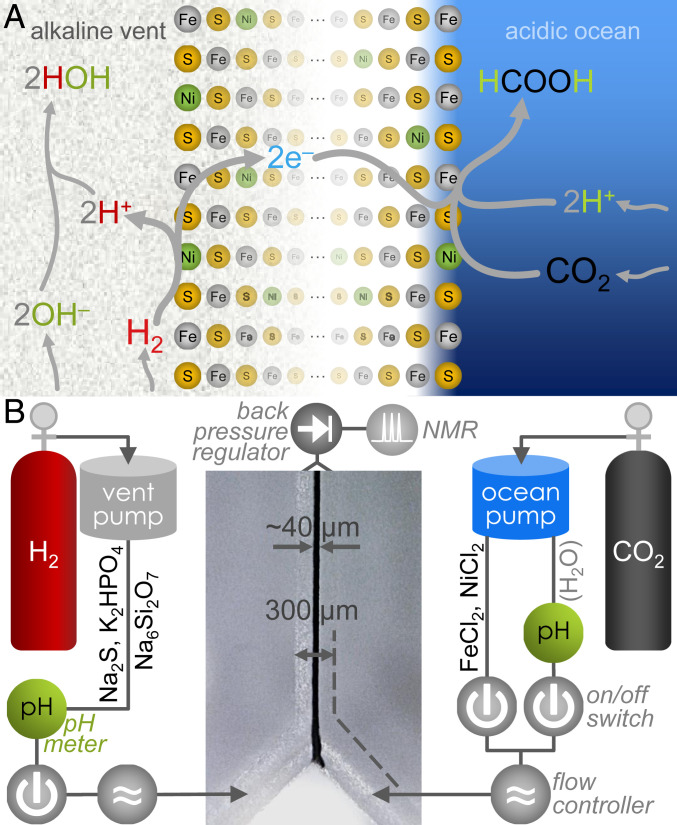
Fig. 1.
Proposed mechanism of pH-driven CO2 reduction with H2 across a conducting Fe(Ni)S barrier, and schematic of the reactor. (A) Under alkaline-vent conditions, the oxidation of H2 (Left) is favored by the alkaline pH due to the presence of hydroxide ions (OH–) that react exergonically in the production of water. Released electrons would travel across the micrometers- to centimeters-thick Fe(Ni)S network (53) (Center) to the more oxidizing acidic solution on the ocean side. There they meet dissolved CO2 and a relatively high concentration of protons (H+), favoring the production of formic acid (HCOOH) or formate (HCOO–). This electrochemical system enables the overall reaction between H2 and CO2, which is not observed under standard reaction conditions. (B) Diagram of the reactor, with embedded micrograph of a reaction run with precipitate at the interface. Further details are provided in the main text and SI Appendix, Fig. S2.