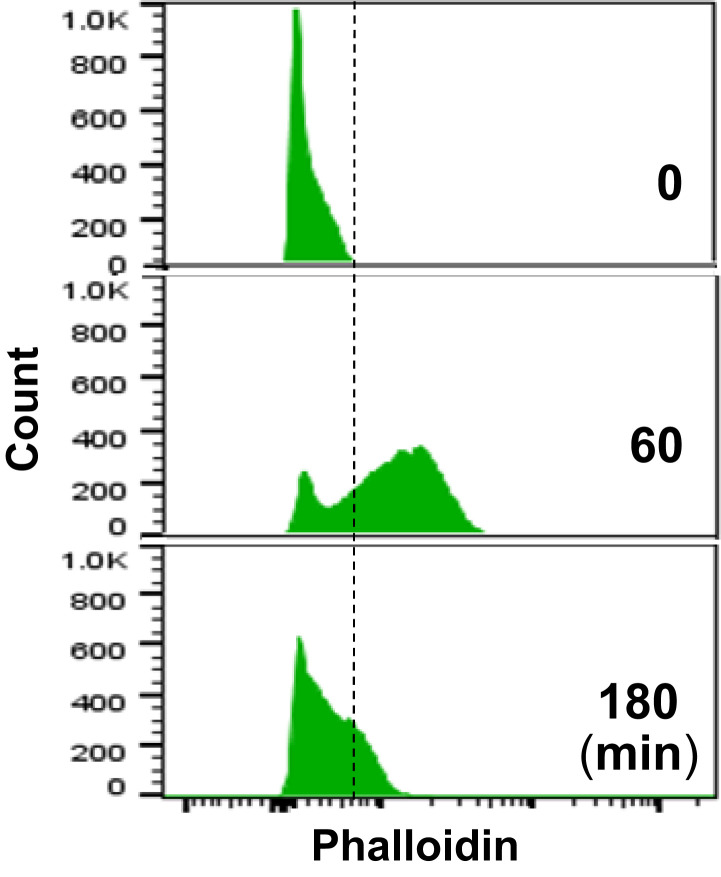
Neutrophil extracellular trap formation (NETosis) is a dynamic process featuring nuclear chromatin extrusion and extracellular trap formation (1) in which ruptures of nuclear envelope and plasma membrane are prerequisite events. Thiam et al. (1) analyze several important cellular events, including cytoskeleton organization, during NETosis using time-lapse microscopy. They conclude that NETosis begins with rapid disassembly of actin cytoskeleton, based on the microscopy analysis of a few hundred neutrophils (1). However, other studies have found that pharmacologic (2, 3) or genetic (4) inhibition of actin assembly decreases NET formation, indicating the role of functional actin cytoskeleton in NETosis induction. To systematically explore the dynamic changes of actin, our time course study with flow cytometry analysis (over 10,000 nonselected neutrophils at each time point) shows that phorbol myristate acetate (PMA) treatment gradually increased actin polymerization, reached the peak (F-actin detected in the majority of, but not all, cells) at 60 min, then gradually decreased to near-baseline levels at 180 min (Fig. 1). Additionally, Metzler et al. (5) reported that neutrophil elastase can degrade F-actin in neutrophils at 30 min after exposure to Candida albicans. These studies suggest that functional actin cytoskeleton may exist for at least 30 min to 60 min in the beginning of NETosis induction.
Fig. 1.
Histograms of flow cytometry analysis of actin polymerization (F-actin detected by RFP-labeled phalloidin) of PMA-treated HL60 cells at 0, 60, and 180 min.
We recently reported that nuclear lamin B is crucial to the nuclear envelop integrity (6), and nuclear envelope rupture and NET formation are driven by PKCα-mediated disassembly of lamin B, which is disassembled into intact full-length molecules, but not fragmented by destructive proteolysis (6). In line with our findings, nuclear lamin A/C is also involved in neutrophil NETosis (7), probably through lamin A/C phosphorylation mediated by cyclin-dependent kinases (CDK) 4/6 (7). Therefore, kinase-mediated nuclear lamina phosphorylation is responsible for nuclear envelope rupture during NETosis.
Nuclear translocation of cytosolic PKCα requires intact cytoskeleton (8), and its association with cytoskeleton (9) is important for PKCα intracellular transportation. In our study, PMA stimulation induces nuclear translocation of cytosolic PKCα, resulting in PKCα nuclear accumulation and the nuclear envelope discontinuation at 60 min (6), which time frame matches with the time course of actin polymerization (Fig. 1), while inhibition of actin assembly attenuates PKCα nuclear accumulation and NETosis. Furthermore, CDK6 is found to be associated with cytoskeleton, and cells from Cdk6 KO mice showed impaired F-actin formation (10), suggesting that CDK6 activation during NETosis (7) may be accompanied by actin polymerization. These studies indicate that functional actin cytoskeleton may be necessary for early-stage nuclear translocation of PKCα (6) and CDK4/6 (7) which are required for nuclear lamina disassembly during NETosis.
As Thiam et al. (1) mention, cortical actin disassembly is required for plasma membrane rupture, which is a known late-stage event for extracellular trap release. Given that chromatin forms the backbone of NETs, release of nuclear chromatin requires rupture of nuclear envelope and plasma membrane. Since actin polymerization and depolymerization are dynamic processes that are involved in different cellular events (10) in activated cells, actin polymerization may be required for nuclear translocation of lamin phosphorylation kinases for nuclear envelope rupture in the early stage (3, 6), while actin disassembly may be necessary for plasma membrane rupture in the late stage of NETosis induction (1).
Footnotes
The author declares no competing interest.
References
- 1.Thiam H. R., et al., NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. U.S.A. 117, 7326–7337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neeli I., Dwivedi N., Khan S., Radic M., Regulation of extracellular chromatin release from neutrophils. J. Innate Immun. 1, 194–201 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neubert E., Meyer D., Kruss S., Erpenbeck L., The power from within − Understanding the driving forces of neutrophil extracellular trap formation. J. Cell Sci. 133, jcs241075 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Stojkov D., et al., ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J. Cell Biol. 216, 4073–4090 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metzler K. D., Goosmann C., Lubojemska A., Zychlinsky A., Papayannopoulos V., A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 8, 883–896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., et al., Nuclear envelope rupture and NET formation is driven by PKCα-mediated lamin B disassembly. EMBO Rep. 2020 Jun, e48779 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amulic B., et al., Cell-cycle proteins control production of neutrophil extracellular traps. Dev. Cell 43, 449–462.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Schmalz D., Kalkbrenner F., Hucho F., Buchner K., Transport of protein kinase C alpha into the nucleus requires intact cytoskeleton while the transport of a protein containing a canonical nuclear localization signal does not. J. Cell Sci. 109, 2401–2406 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Niggli V., Djafarzadeh S., Keller H., Stimulus-induced selective association of actin-associated proteins (alpha-actinin) and protein kinase C isoforms with the cytoskeleton of human neutrophils. Exp. Cell Res. 250, 558–568 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara I., Takahashi S., Tadakuma H., Funatsu T., Ishiwata S., Microscopic analysis of polymerization dynamics with individual actin filaments. Nat. Cell Biol. 4, 666–673 (2002). [DOI] [PubMed] [Google Scholar]