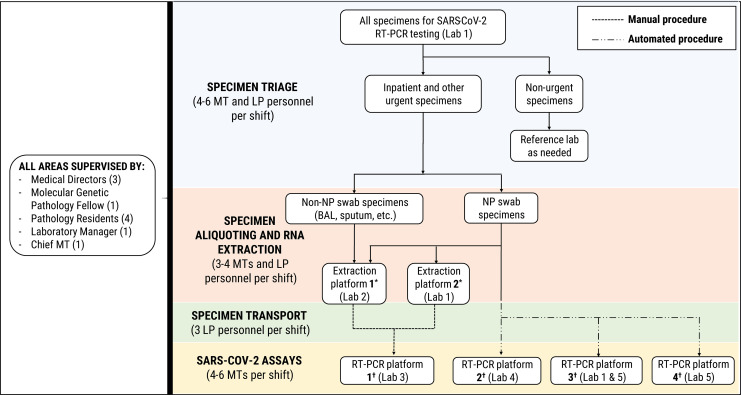
Figure 4.
Specimen triage and distribution of the analytic workflow for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) molecular diagnostic testing performed on various RT-PCR platforms in the molecular diagnostics laboratory at Houston Methodist Hospital. Locations of various instruments within 5 separate laboratory areas are denoted by (Lab #). (*) RNA extraction platforms include EZ1 advanced XL (Qiagen) and qiasymphony SP (Qiagen). (†) SARS-CoV-2 RT-PCR assay platforms include (1) ABI 7500 fastdx (Applied Biosystems), (2) panther fusion (Hologic, Inc), (3) genexpert infinity (Cepheid) and genexpert Dx GX-IV (Cepheid), and (4) filmarray 2.0 (BioFire Defense, LLC). BAL indicates bronchoalveolar lavage; LP, labor pool personnel; MT, medical technologist; NP, nasopharyngeal; RT-PCR, reverse transcription polymerase chain reaction.