Figure 5.
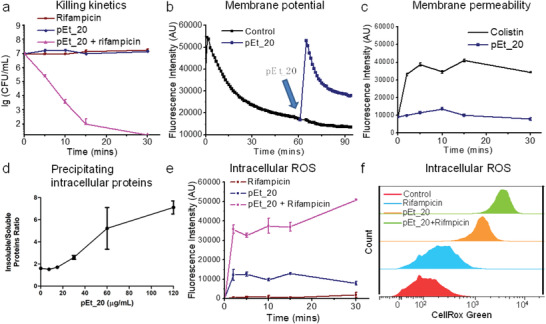
Mechanistic study of combination treatment using pEt_20 and rifampicin. a) Rapid killing kinetics of the combination (>99.9% killing in 10 min). pEt_20: 0.5× MIC (7.8 µg mL−1), rifampicin: 0.5× MIC (0.50 µg mL−1). Limit of detection: 50 CFU mL−1. b) Analysis of membrane potential of A. baumannii 1709 using DiSC3, a cationic fluorescent dye that accumulates onto negatively charged bacterial membrane through electrostatic interaction, quenching fluorescence. pEt_20 interacted with the phosphate groups on the membrane through strong bidendate hydrogen‐bonding interaction, which released DiSC3, increasing fluorescence intensity. After membrane translocation of pEt_2016, the phosphate groups were released for binding DiSC3, decreasing fluorescence intensity. pEt_20: 7.8 µg mL−1. c) Fluorescence of bacteria treated with pEt_20 and PI dye, which only stained bacteria with damaged membrane. Colistin sulfate significantly increased membrane permeability, while pEt_20 did not exert a significant effect (pEt_20 and colistin sulfate at 0.5× MIC, 7.8 and 0.50 µg mL−1, respectively). This finding is in agreement with our previous study that the pEt_20 translocated membrane instead of lysing it.[ 16 ] d) Soluble and insoluble protein quantification following polymer treatment. Log‐phase culture of A. baumannii 1709 was treated with increasing concentration of pEt_20 for 15 min, resulting in an increase in insoluble/soluble protein ratio. The polymer treatment caused bacterial protein precipitation in a dose‐dependent manner. Data shown are the results of two independent biological replicates carried out in technical triplicates. e) Fluorescence intensity analysis of intracellular ROS probe CellRox Green in A. baumannii BAA‐1709 (≈107 CFU mL−1) after treatment with rifampicin (0.50 µg mL−1), pEt_20 (7.8 µg mL−1), and their combination (pEt_20: 7.8 µg mL−1; rifampicin: 0.50 µg mL−1) over various periods of time. f) Flow cytometry histograms. The results from (e,f) show that the combination significantly enhanced intracellular ROS generation. This might be responsible for the rapid bacteria killing of the combination under the same treatment conditions in a. pEt_20 translocated bacterial membrane followed by binding cytosolic proteins or genes (Figure S4, Supporting Information), facilitating ROS generation and thus killing the bacteria.
