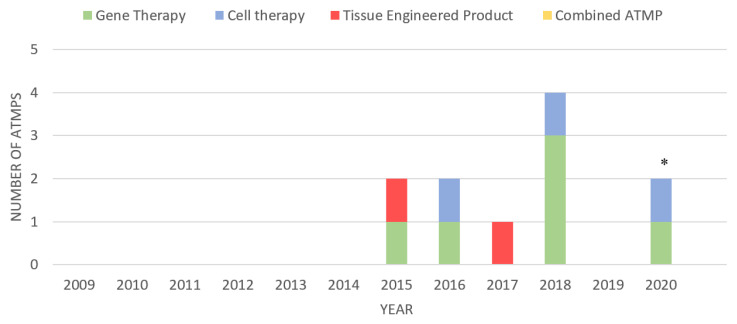
Figure 1.
ATMPs (advanced therapy medicinal products; gene therapies, cell therapies, tissue-engineered products and combined ATMPs) that have been granted marketing approval by the EMA. Note: * indicates cell therapy “KTE-X19” expected to receive market authorization in 2020 (the EMA has validated their application but it currently under review [8,9]).