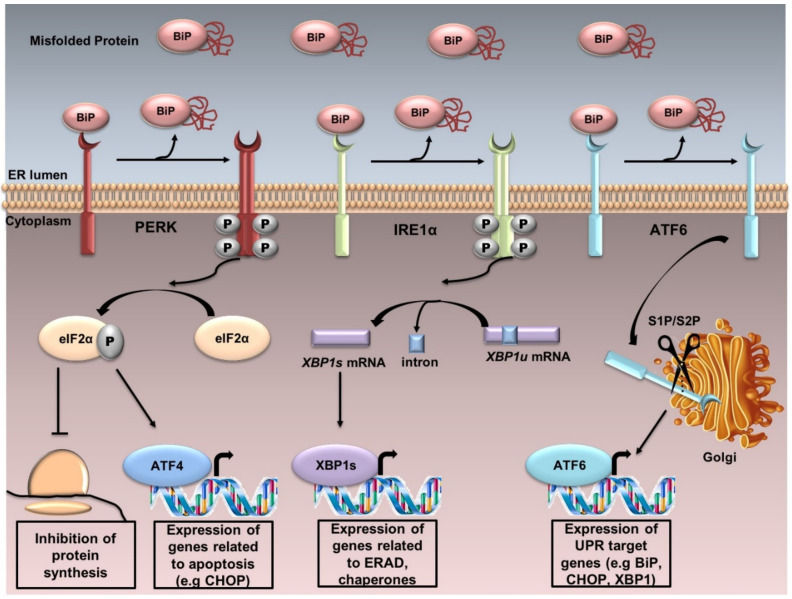
Figure 1.
The UPR. UPR is controlled by three sensor proteins: PERK, IRE1α and ATF6. Under normal conditions, these proteins are associated with BiP and thus remain inactive. Under ER stress, BiP is released from these sensor proteins, and UPR cascade is activated after further dimerization and autophosphorylation of PERK and IRE1α, and regulated intramembrane proteolysis of ATF6. PERK activation induces the phosphorylation of eIF2α. Phosphorylated eIF2α reduces the global protein synthesis and promotes the translation of the activating transcription factor 4 (ATF4) which upregulates the genes related to apoptosis. IRE1α dimerizes, autotransphosphorylates, and its endoribonuclease activity enables the splicing of the unspliced X-box binding protein 1 XBP1u to the spliced XBP1s. XBP1s upregulates the transcription of several genes involved in UPR and ERAD. ATF6 is localized at the ER in unstressed cells. Under ER stress, ATF6 translocates from the ER to the Golgi, where it is cleaved sequentially by the enzymes site 1 protease (S1P) and site 2 protease (S2P). Active ATF6 is transferred into the nucleus, where it binds the promoters of several genes involved in UPR (such as C/EBPα-homologous protein (GADD153 or CHOP), BiP, XBP1).