Abstract
Fabry disease is an X-linked lysosomal storage disorder caused by mutations in the GLA gene encoding the α-galactosidase A enzyme. This enzyme cleaves the last sugar unit of glycosphingolipids, including globotriaosylceramide (Gb3), globotriaosylsphingosine (lyso-Gb3), and galabiosylceramide (Ga2). Enzyme impairment leads to substrate accumulation in different organs, vascular endothelia, and biological fluids. Enzyme replacement therapy (ERT) is a commonly used treatment. Urinary analysis of Gb3 isoforms (different fatty acid moieties), as well as lyso-Gb3 and its analogues, is a reliable way to monitor treatment. These analogues correspond to lyso-Gb3 with chemical modifications on the sphingosine moiety (−C2H4, −C2H4+O, −H2, −H2+O, +O, +H2O2, and +H2O3). The effects of sample collection time on urinary biomarker levels between ERT cycles were not previously documented. The main objective of this project was to analyze the aforementioned biomarkers in urine samples from seven Fabry disease patients (three treated males, three treated females, and one ERT-naïve male) collected twice a day (morning and evening) for 42 days (three ERT cycles). Except for one participant, our results show that the biomarker levels were generally more elevated in the evening. However, there was less variability in samples collected in the morning. No cyclic variations in biomarker levels were observed between ERT infusions.
Keywords: Fabry disease, diurnal variation, globotriaosylceramide, globotriaosylsphingosine, mass spectrometry, glycosphingolipids
1. Introduction
Fabry disease (FD) (OMIM no. 301500) is an X-linked monogenic lysosomal storage disorder. While males are often more affected than females, some females can be as severely affected as males due to X-chromosome inactivation [1]. The main clinical features of FD are cardiac hypertrophy, progressive renal deficiency, and increased risk of ischemic strokes. Other signs and symptoms include acroparesthesia, hypohidrosis, gastrointestinal involvement, angiokeratomas, cornea verticillata [2], and vascular tortuosities of the upper eyelid [3]. FD is caused by mutations in the GLA gene encoding the α-galactosidase A enzyme (EC 3.2.1.22). The function of this glycoside hydrolase is to cleave the terminal α-galactosyl moieties from glycoproteins or glycolipids [1]. The deficiency of the enzyme leads to the accumulation of substrates such as globotriaosylsphingosine (lyso-Gb3), galabiosylceramide (Ga2), and globotriaosylceramide (Gb3) in cells [4], tissues [5], and biological fluids [6,7,8,9]. Different untargeted metabolomic studies performed in our laboratory with urine and plasma specimens from FD patients identified different analogues of lyso-Gb3 [10,11], as well as analogues/isoforms of Ga2 [12] and Gb3 [13], as FD biomarkers. The analogues correspond to molecules with modified sphingosine moieties, whereas the isoforms correspond to molecules with different fatty acid chains. As for lyso-Gb3 in urine, the following modifications on the sphingosine moiety were detected: −C2H4 (−28 Da), −C2H4+O (−12 Da), −H2 (−2 Da), −H2+O (+14 Da), +O (+16 Da), +H2O2 (+34 Da), and +H2O3 (+50 Da) [10]. In fact, most of the lyso-Gb3 analogues in urine are more abundant than lyso-Gb3 itself. Previous results showed that FD children with the late-onset cardiac variant p.N215S mutation presented normal urinary levels of lyso-Gb3, but abnormal levels of some lyso-Gb3 analogues [14]. Similarly, five patients with the p.N215S mutation presented abnormal levels of methylated Gb3 isoforms, but normal levels of non-methylated Gb3 isoforms [7]. Moreover, the analysis of urine samples from FD patients with the late-onset IVS4+919G > A cardiac variant mutation prevalent in Taiwan revealed that some lyso-Gb3 analogue levels had a positive association with the left-ventricular mass index and/or the Mainz Severity Score Index [15]. Chaperone therapy with migalastat (Galafold, Amicus Therapeutics) is now available for patients with amenable mutations [16]. However, the most common treatment for FD remains ERT [17], which consists of bi-weekly intravenous infusions of recombinant α-galactosidase A such as agalsidase-alpha at 0.2 mg/kg (Replagal, Shire/Takeda Pharmaceutical) or agalsidase-beta at either 1.0 or 0.3 mg/kg (Fabrazyme, Sanofi Genzyme). A gene therapy clinical trial for FD is also ongoing [18]. The analysis of biomarkers in urine is a method of choice for monitoring the response of FD patients to ERT since sample collection is non-invasive. However, to the best of our knowledge, there are no published studies investigating the diurnal variation of urinary biomarkers and their fluctuation between ERT infusions. The main objectives of this research project were, thus, to evaluate if the urinary FD biomarker concentrations show statistically significant differences when sampled in the morning compared to the evening, and if the levels fluctuate periodically between ERT infusions using bi-daily monitoring over three ERT cycles (42 days).
2. Results
2.1. Biomarker Measures according to the Collection Time
2.1.1. Mean Measured Biomarker Levels
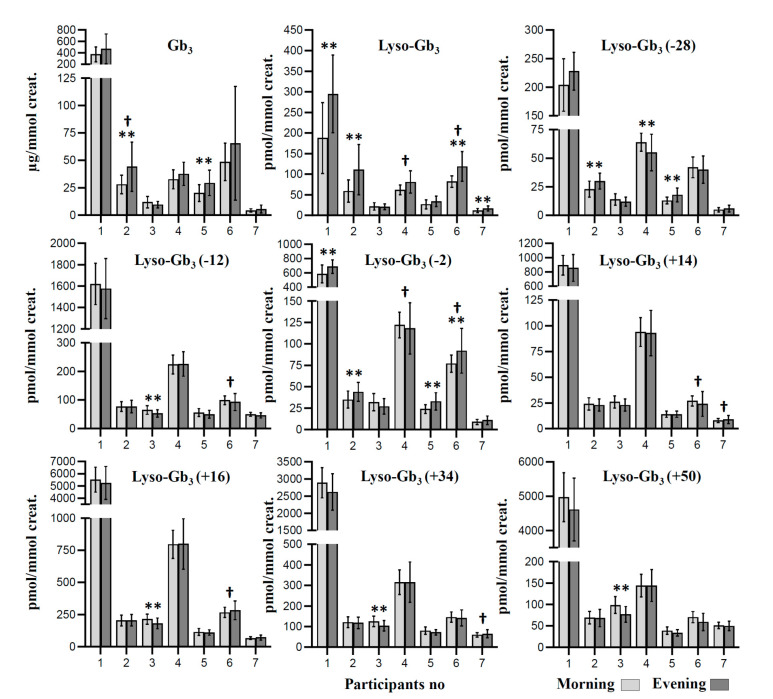
Raw data are available in Supplemental Table S1. FD biomarkers are expressed as mean measured levels for each collection time point (Figure 1). Paired-sample t-tests were used to determine whether there was a statistically significant mean difference between the biomarker concentrations measured in specimens collected in the morning compared to specimens collected in the evening. Results are presented in Table 1 for each participant, along with a mean ± one standard deviation for each collection time. Results show that the measured concentrations might be influenced by the collection time for some biomarkers. Our results show a statistically significant mean difference for lyso-Gb3 levels in four participants (1, 2, 6, and 7), where concentrations were higher in urine specimens collected in the evening compared to those collected in the morning. Similar results were obtained for lyso-Gb3 analogue (−2 Da) in participants 1, 2, 5, and 6, and for Gb3 in participants 2 and 5. Regarding lyso-Gb3 analogues (−12 Da), (+14 Da), (+16 Da), (+34 Da), and (+50 Da), there were no mean differences in the measured levels based on the collection time, except for patient 3, where the measured values had a tendency to be higher in morning specimens. Finally, there were mixed results for the lyso-Gb3 analogue (−28 Da), where the measured values were higher in the evening for participants 2 and 5, but higher in the morning for participant 4.
Figure 1.
Mean concentration of Fabry biomarkers for three enzyme replacement therapy (ERT) cycles (n = 42 days) at two different collection times in seven study participants. All participants were under ERT, except participant no. 1 who never received treatment. Patients 1–4 were males, whereas patients 5–7 were females. For lyso-Gb3 analogues, the mass difference in Da compared to lyso-Gb3 is indicated in brackets. ** corrected p-value ≤ 0.001 for the paired-sample t-test; † corrected p-value ≤ 0.001 for Levene’s test for equality of variances.
Table 1.
Paired-sample t-tests assessing mean differences in measured biomarker levels over 42 days in seven Fabry patients at two different collection time points (morning and evening). Means ± standard deviation are shown. For statistically significant results, p-values are highlighted in gray or in black if the highest mean concentration value was measured in the morning or the evening, respectively.
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | Participant 6 | Participant 7 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | |
| Gb3 | 373 ± 128 | 468 ± 261 | <0.050 | 28 ± 8 | 44 ± 23 | <0.001 † | 12 ± 5 | 10 ± 3 | <0.050 | 33 ± 9 | 38 ± 11 | <0.050 | 20 ± 8 | 29 ± 12 | <0.001 † | 49 ± 17 | 66 ± 52 | 0.059 | 4 ± 2 | 6 ± 4 | 0.060 |
| Lyso-Gb3 | 188 ± 86 | 295 ± 94 | <0.001 † | 59 ± 27 | 111 ± 61 | <0.001 † | 22 ± 9 | 21 ± 7 | 0.525 | 62 ± 12 | 81 ± 27 | <0.050 | 27 ± 11 | 34 ± 13 | <0.050 | 82 ± 14 | 119 ± 36 | <0.001 † | 12 ± 5 | 17 ± 6 | <0.001 † |
| Lyso-Gb3 (−28) | 204 ± 46 | 228 ± 33 | <0.050 | 23 ± 7 | 30 ± 7 | <0.001 † | 14 ± 5 | 12 ± 4 | <0.050 | 64 ± 8 | 55 ± 16 | 0.001 † | 13 ± 3 | 18 ± 6 | <0.001 † | 42 ± 9 | 40 ± 12 | 0.224 | 5 ± 2 | 6 ± 3 | 0.208 |
| Lyso-Gb3 (−12) | 1620 ± 193 | 1576 ± 281 | 0.282 | 77 ± 17 | 77 ± 22 | 0.894 | 65 ± 15 | 53 ± 13 | <0.001 † | 224 ± 33 | 226 ± 43 | 0.831 | 56 ± 14 | 50 ± 15 | <0.050 | 99 ± 15 | 93 ± 30 | 0.224 | 50 ± 7 | 46 ± 9 | <0.050 |
| Lyso-Gb3 (−2) | 585 ± 126 | 689 ± 95 | <0.001 † | 35 ± 10 | 44 ± 11 | <0.001 † | 32 ± 10 | 27 ± 9 | <0.050 | 122 ± 15 | 118 ± 30 | 0.393 | 24 ± 5 | 33 ± 10 | <0.001 † | 77 ± 10 | 92 ± 26 | 0.001 † | 9 ± 3 | 11 ± 5 | 0.135 |
| Lyso-Gb3 (+14) | 894 ± 138 | 855 ± 188 | <0.050 | 24 ± 6 | 23 ± 6 | 0.105 | 26 ± 6 | 23 ± 6 | <0.050 | 94 ± 14 | 93 ± 22 | 0.457 | 14 ± 3 | 14 ± 3 | 0.768 | 27 ± 5 | 24 ± 12 | 0.280 | 8 ± 2 | 9 ± 4 | 0.457 |
| Lyso-Gb3 (+16) | 5524 ± 1017 | 5246 ± 1351 | <0.050 | 204 ± 43 | 206 ± 45 | 0.760 | 215 ± 39 | 182 ± 42 | <0.001 † | 797 ± 109 | 800 ± 196 | 0.927 | 115 ± 27 | 110 ± 21 | 0.279 | 267 ± 40 | 283 ± 73 | 0.234 | 67 ± 11 | 72 ± 20 | 0.094 |
| Lyso-Gb3 (+34) | 2892 ± 440 | 2622 ± 532 | 0.001 | 121 ± 26 | 118 ± 28 | 0.527 | 125 ± 25 | 104 ± 26 | <0.001 † | 315 ± 60 | 316 ± 98 | 0.797 | 80 ± 18 | 72 ± 13 | <0.050 | 146 ± 25 | 142 ± 39 | 0.609 | 60 ± 11 | 65 ± 20 | 0.137 |
| Lyso-Gb3 (+50) | 4973 ± 711 | 4613 ± 913 | <0.050 | 69 ± 15 | 68 ± 20 | 0.779 | 99 ± 20 | 77 ± 18 | <0.001 † | 144 ± 27 | 144 ± 37 | 0.847 | 39 ± 9 | 34 ± 8 | <0.050 | 70 ± 13 | 59 ± 20 | <0.050 | 51 ± 7 | 50 ± 11 | 0.583 |
† indicates that statistical significance was still valid after correction for multiple comparisons using the Holm–Šídák procedure (63 comparisons); M: morning collection; E: evening collection. All participants were under ERT except for participant no. 1 who was an ERT-naïve patient. Patients 1–4 were males, whereas patients 5–7 were females. For lyso-Gb3 analogues, the mass difference in Da compared to lyso-Gb3 is indicated in brackets.
2.1.2. Variance of Biomarker Measures
Levene’s test for equality of variances was used to verify the homogeneous variance of biomarker levels when urine specimens were collected either in the morning or in the evening. Results are shown in Table 2 for each collection time, along with relative standard deviations (RSDs) (n = 42 days). In general, RSDs were higher for specimens collected in the evening. The variance was equivalent for the morning and evening specimens for each biomarker according to Levene’s test for participants 1, 3, and 5. However, Gb3 levels had more variance in evening specimens for participant 2, while lyso-Gb3 and the analogue (−2 Da) levels had more variance in evening specimens for participant 4. For participant 6, our results show that five biomarkers (lyso-Gb3, and analogues (−12 Da), (−2 Da), (+14 Da), and (+16 Da)) had a higher variance for specimens collected in the evening. The same tendency was observed for lyso-Gb3 analogues (+14 Da) and (+34 Da) for participant 7.
Table 2.
Levene’s test for equality of variances examining the influence of urine collection time on the variability of the measured biomarker levels over 42 days in seven Fabry patients. Relative standard deviations (RSDs) are shown (n = 42) for each collection time. For statistically significant results, p-values are highlighted in gray or in black if the highest variance was obtained in the morning or in the evening, respectively.
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | Participant 6 | Participant 7 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %RSD | %RSD | %RSD | %RSD | %RSD | %RSD | %RSD | |||||||||||||||
| M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | M | E | p | |
| Gb3 | 34 | 56 | 0.190 | 30 | 51 | <0.001 † | 44 | 31 | 0.101 | 27 | 28 | 0.089 | 38 | 40 | 0.495 | 35 | 79 | <0.050 | 37 | 65 | <0.050 |
| Lyso-Gb3 | 46 | 32 | 0.813 | 46 | 55 | <0.050 | 41 | 32 | 0.247 | 20 | 33 | <0.001 † | 39 | 37 | 0.448 | 17 | 30 | <0.001 † | 38 | 37 | 0.118 |
| Lyso-Gb3 (−28) | 23 | 15 | 0.126 | 30 | 25 | 0.614 | 33 | 32 | 0.170 | 13 | 29 | <0.050 | 26 | 33 | <0.050 | 21 | 31 | 0.137 | 33 | 53 | <0.050 |
| Lyso-Gb3 (−12) | 12 | 18 | 0.156 | 22 | 29 | 0.227 | 23 | 24 | 0.562 | 15 | 19 | 0.538 | 26 | 29 | 0.889 | 15 | 32 | <0.001 † | 14 | 19 | 0.738 |
| Lyso-Gb3 (−2) | 22 | 14 | 0.079 | 28 | 24 | 0.790 | 30 | 32 | 0.426 | 12 | 25 | <0.001 † | 22 | 30 | <0.050 | 14 | 28 | <0.001 † | 32 | 48 | <0.050 |
| Lyso-Gb3 (+14) | 15 | 22 | 0.277 | 23 | 26 | 0.696 | 22 | 25 | 0.708 | 14 | 24 | 0.189 | 20 | 24 | 0.903 | 18 | 49 | <0.001 † | 22 | 42 | <0.001 † |
| Lyso-Gb3 (+16) | 18 | 26 | 0.300 | 21 | 22 | 0.691 | 18 | 23 | 0.881 | 14 | 24 | 0.183 | 23 | 20 | 0.317 | 15 | 26 | <0.001 † | 17 | 28 | <0.050 |
| Lyso-Gb3 (+34) | 15 | 20 | 0.783 | 21 | 24 | 0.917 | 20 | 25 | 0.776 | 19 | 31 | 0.170 | 23 | 18 | <0.050 | 17 | 27 | <0.050 | 18 | 31 | <0.001 † |
| Lyso-Gb3 (+50) | 14 | 20 | 0.285 | 21 | 30 | 0.150 | 20 | 24 | 0.308 | 18 | 26 | 0.536 | 23 | 22 | 0.056 | 18 | 34 | 0.063 | 14 | 22 | <0.050 |
† indicates that statistical significance was still valid after correction for multiple comparisons using the Holm–Šídák procedure (63 comparisons); M: morning collection; E: evening collection; RSD: relative standard deviation. For lyso-Gb3 analogues, the mass difference in Da compared to lyso-Gb3 is indicated in brackets.
2.2. Longitudinal Follow-Up
2.2.1. Untreated Fabry Disease Participant
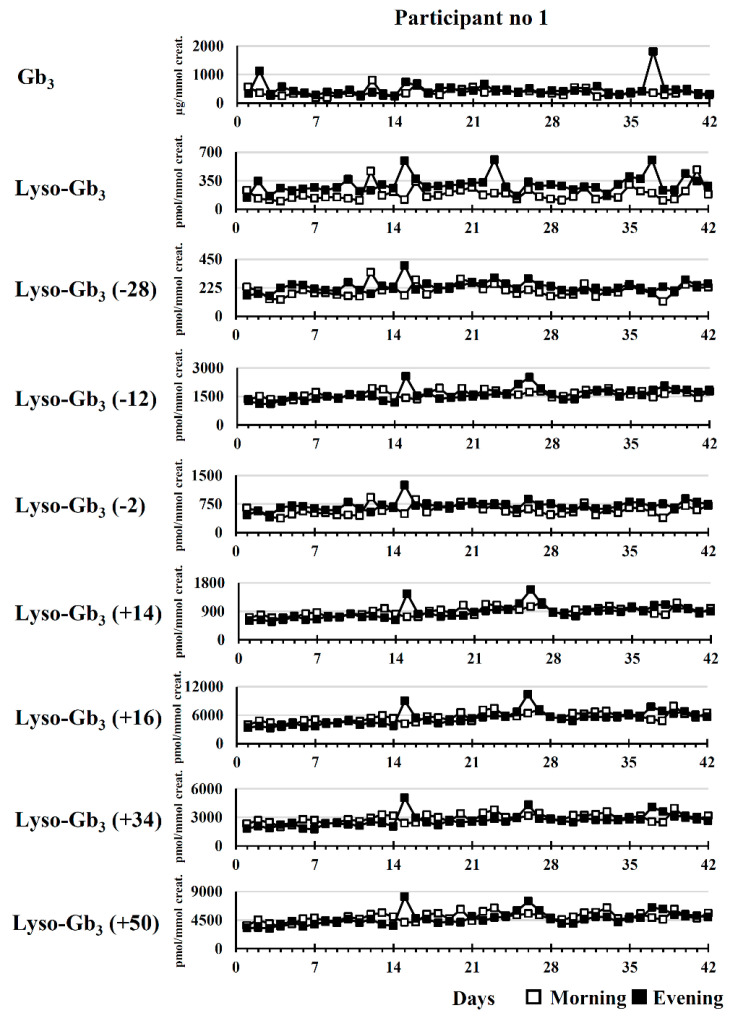
The longitudinal biomarker follow-up of participant no. 1 was useful to evaluate the variability of the measured biomarker concentrations in urine specimens from an untreated Fabry patient. Longitudinal follow-ups are shown in Figure 2. According to RSDs, the dispersion of the measured concentrations (n = 42) was higher for lyso-Gb3 (morning RSD: 46%; evening RSD: 32%) and Gb3 (morning RSD: 34%; evening RSD: 56%) compared to lyso-Gb3 analogues with RSDs ≤ 23% in urine specimens collected in the morning, and RSDs ≤ 26% in urine specimens collected in the evening.
Figure 2.
Longitudinal variation (n = 42) of Fabry biomarker levels in a 30-year-old untreated male (no ERT) (participant no. 1). For lyso-Gb3 analogues, the mass difference in Da compared to lyso-Gb3 is indicated in brackets.
2.2.2. Participants Treated with ERT
Considering that biomarker levels had equivalent or lower variance in urine specimens collected in the morning compared to urine specimens collected in the evening, results of longitudinal biomarker follow-ups in treated males and females are shown in specimens collected in the morning only.
Male Fabry Disease Participants
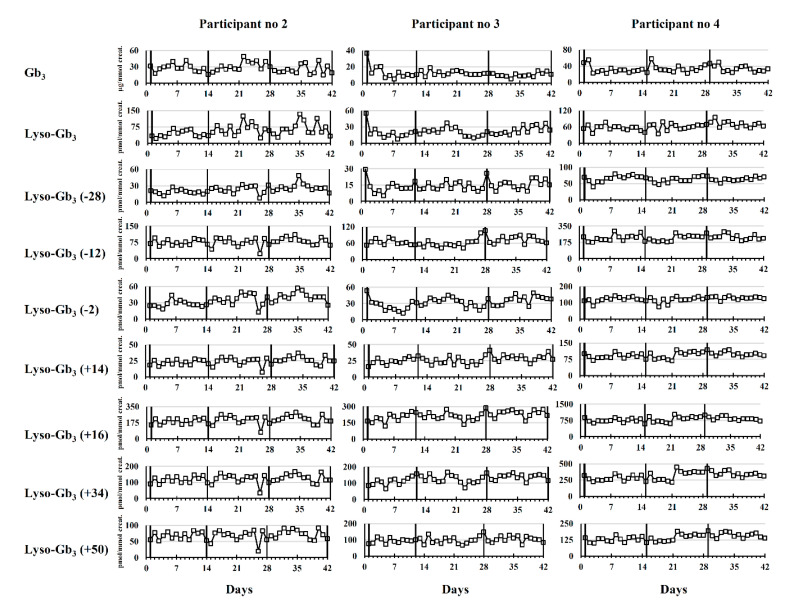
Longitudinal biomarker follow-ups are shown in Figure 3 for ERT-treated male participants 2, 3, and 4.
Figure 3.
Longitudinal variation (n = 42) of Fabry disease biomarker levels during three ERT cycles in male patients. Results are shown for morning urine collection only. For lyso-Gb3 analogues, the mass difference in Da compared to lyso-Gb3 is indicated in brackets. Vertical lines indicate the time points where Fabry patients received their ERT infusions.
Visual inspection of longitudinal follow-ups in Figure 3 suggests that, in general, the biomarker concentrations were stable in male patients during the study and oscillated around a mean biomarker value. In fact, no clear pattern of cyclic variation was observed in relation to ERT infusions. A visual examination of longitudinal follow-ups also showed that lyso-Gb3 analogues (−12 Da), (+14 Da), (+16 Da), (+34 Da), and (+50 Da) had similar profiles.
Female Fabry Disease Participants
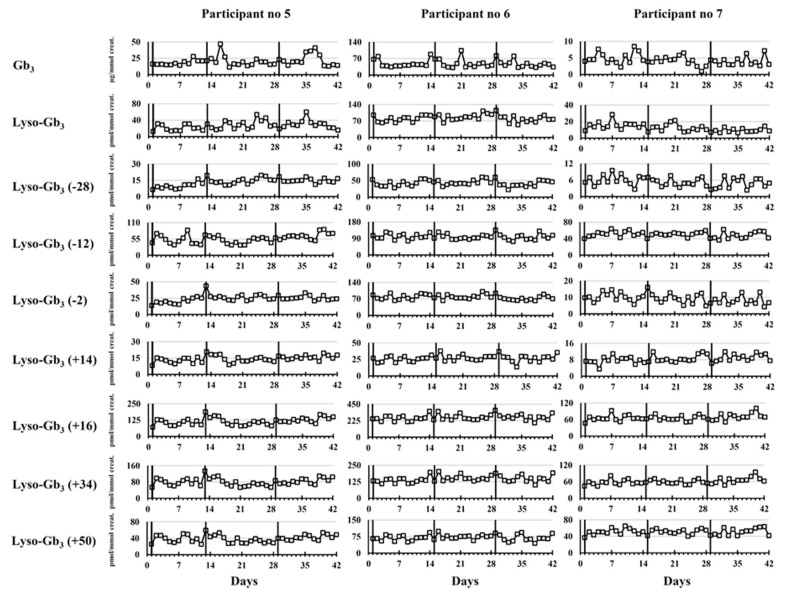
Longitudinal biomarker follow-ups are shown in Figure 4 for female participants 5, 6, and 7 who were all ERT-treated.
Figure 4.
Longitudinal variation (n = 42) of Fabry disease biomarker levels during three ERT cycles in female patients. Results are shown for morning urine collection only. The vertical lines indicate the time points where Fabry patients received their ERT infusions. For lyso-Gb3 analogues, the mass difference in Da compared to lyso-Gb3 is indicated in brackets.
Our results show that there was no clear pattern of cyclic biomarker variation observed in urine specimens of female participants during three cycles of ERT infusions.
3. Discussion
The aim of the present study was to evaluate the variation of FD biomarkers, such as Gb3, lyso-Gb3, and related analogues, in urine specimens collected by seven participants diagnosed with FD (one untreated and six ERT-treated). Urine specimens were collected daily, at two different timepoints, for a duration of 42 days. The collection time (morning or evening urine specimens) and the administration of ERT infusions were evaluated as potential variability factors. A total of 84 urine specimens were collected at home by each study participant. Patient compliance was excellent as no urine specimen collection was missed. Two shipments with thawed urine specimens were received from participant no. 3 (day 26 to day 42). However, this was not an issue considering that Gb3 and lyso-Gb3 and related analogues are stable for at least 24 h at room temperature in urine specimens [7,9]. Urine specimens offer many advantages for biomarker analyses. Collection is safe and can be performed in a non-invasive way by patients, without the need for trained personnel such as phlebotomists. The analysis of spot urine specimens is more convenient compared to 24-h urine collection specimens, but results can vary according to the rate of urine production. Creatinine, a compound excreted at a constant rate in urine through glomerular filtration [19], is commonly used to normalize results, which are then compared with age- and gender-matched reference values. Gb3 normalization with creatinine might be arguable, considering that its presence in urine does not originate from glomerular filtration, but from the shedding of renal tubular cells and podocytes in urine [20]. Consequently, Gb3 normalization with membrane lipids such as sphingomyelin or phosphatidylcholine was previously investigated. Mills and colleagues [21] showed that the distribution of Gb3-to-sphingomyelin ratios was similar to the distribution of the Gb3-to-creatinine ratios. Whitfield and colleagues had the same conclusions regarding phosphatidylcholine [22]. Nevertheless, precautions should always be taken for the analysis of Gb3 in urine since urine specimens should be homogenized carefully, and filter papers impregnated with urine should be saturated and dried horizontally to avoid unequal distribution of Gb3 and creatinine molecules.
Results obtained following the paired-sample t-tests show that the collection time might influence the biomarker levels in some cases. The measured values were generally more elevated in specimens collected in the evening, except for participant no. 3. Interestingly, there was no difference in lyso-Gb3 analogue (+14 Da) urinary levels depending on the collection time in all participants. This might indicate that this specific biomarker could be useful for long-term follow-up when it is not possible to collect urine specimens at a specific time. The results obtained following Levene’s test for equality of variances revealed that there was usually more biomarker level variability in the evening urine specimens. Specimens collected in the evening might be more prone to variations due to external factors such as physical activity, food intake, and water consumption. The results also showed that the concentrations of urinary lyso-Gb3 and Gb3 measured longitudinally were subject to more variability in the untreated patient than the measured concentrations of lyso-Gb3 analogues, according to RSDs (n = 42 days). A similar trend was observed in treated patients. This might indicate that the urinary levels of lyso-Gb3 analogues are more reliable and less variable for long-term follow-up and monitoring of FD patients than Gb3 and lyso-Gb3 itself. Regarding the variability of biomarker concentrations between ERT infusions, we initially hypothesized a cyclic biomarker variation with reduced biomarker levels immediately after infusion, followed by a slow increase over the rest of the 14-day cycle. A visual examination of Figure 3 and Figure 4 does not corroborate this initial hypothesis. Considering these results, urine specimens collected in the morning should be preferred over urine specimens collected in the evening for the quantification of FD biomarkers as part of a longitudinal evaluation. The results obtained do not suggest that urine specimens need to be collected at a specific time between two ERT infusions, since this was not a factor of variability in the present study. Urinary lyso-Gb3 analogues might be more reliable for longitudinal follow-up compared to lyso-Gb3 and Gb3, considering that some of these analogues showed less variability depending on the collection time than lyso-Gb3 or Gb3. It is noteworthy to mention that the analysis of lyso-Gb3 analogues is particularly relevant in urine, considering that lyso-Gb3 represents only approximately 2% of all lyso-Gb3-related species in this matrix. In comparison, lyso-Gb3 represents approximately 57% of all lyso-Gb3-related species in plasma [8,9].
4. Conclusions
According to the results obtained in this study, there is no cyclic variation of the urinary Fabry disease biomarkers (Lyso-Gb3 and analogues, and Gb3) normalized to creatinine between two ERT cycles. The diurnal variation observed for the urinary Fabry disease biomarkers normalized to creatinine might be attributed not only to the sphingolipid levels, but also to the variation in the creatinine levels. Nevertheless, the biomarker/creatinine ratios show less variation in urine specimens collected in the morning. Moreover, the lyso-Gb3 analogue/creatinine ratios show less variation than the lyso-Gb3/creatinine and Gb3/creatinine ratios. Finally, this study suggests that the urinary Fabry disease biomarker/creatinine ratios are more elevated in specimens collected in the evening except for the lyso-Gb3 analogue (+14 Da) which shows similar abundance at both collection times.
5. Materials and Methods
5.1. Ethics Approval
This multicenter research project was approved by the Research Ethics Board (REB) at the Faculty of Medicine and Health Sciences at the Centre Intégré Universitaire de Santé et de Services Sociaux de l’Estrie-Centre Hospitalier Universitaire de Sherbrooke (CIUSSSE-CHUS) (REB no 06-011, Approval date: 22 February 2006) and at the CIUSSS du Nord-de-l’Île-de-Montréal-Hôpital du Sacré-Coeur de Montréal (REB no 2014-1071, Approval date: 16 October 2014). All subjects provided informed consent for inclusion before their participation in the study. The study was conducted in accordance with the Declaration of Helsinki.
5.2. Recruitment of Participants
Seven FD patients who were confirmed either by demonstrating a marked enzyme deficiency of α-galactosidase A and/or via GLA gene mutation analysis. They were recruited from two centers in the province of Quebec: CIUSSSE-CHUS (Sherbrooke, QC, Canada) and CIUSSS du Nord-de-l’Île-de-Montréal-Hôpital du Sacré-Coeur de Montréal (Montreal, QC, Canada). Participant demographics are shown in Table 3. All participants, except one, were receiving ERT.
Table 3.
Participant demographics. n/a: not applicable; ERT: enzyme replacement therapy; eGFR: estimated glomerular filtration rate; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
| Patient No | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | F | F | F |
| Age (years) | 30 | 28 | 40 | 36 | 49 | 57 | 67 |
| Treatment status | No treatment | Replagal | Fabrazyme | Fabrazyme | Replagal | Replagal | Fabrazyme |
| Mutation | c.17_327del | c.612G > A | c.35_47del13 | c.1241T > C | c.1042G > C | c.1241T > C | c.877C > A |
| ERT start date | n/a | 2005-07-26 | 2008-09-30 | 2003-05-31 | 2009-01-14 | 2011-02-02 | 2003-04-15 |
| Serum creatinine (µmol/L) | 97 | 78 | 97 | 96 | 57 | 76 | 92 |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 91 | 116 | 83 | 87 | 105 | 76 | 56 |
| Urinary proteins (g/24 h) | 1.07 | 0.14 | 1.19 | 1.38 | 0.25 | 3.21 | 0.07 |
5.3. Urine Specimen Collection
Urine specimen collection was performed at home by participants. Each participant received the following urine collection kit: 86 conical centrifuge tubes in polypropylene (50 mL) divided into four Styrofoam racks, four pre-addressed shipping boxes, eight icepacks, parafilm, and four large plastic bags. Two urine specimens (identified as M and E) were collected each day for a duration of 42 days, representing three ERT cycles for treated patients. Specimen M was collected in the morning (before breakfast), and specimen E was collected in the evening, after dinner. The collection of the first urine specimen was performed when ERT-treated patients received their day-1 infusion. Parafilm was used to seal the cap of the tube after urine collection, and the tube was frozen immediately. Collected urine specimens (n = 25) were placed in a Styrofoam rack and inserted in a large plastic bag in a pre-addressed box containing two icepacks. Samples were picked up by a shipping courier and delivered to our laboratory in Sherbrooke, QC for analysis.
5.4. Analysis of Fabry Disease Biomarkers
FD biomarkers were analyzed as mentioned below using assays previously devised and validated in our laboratory. Intra-assay and inter-assay precisions were good with relative standard deviations (RSDs) ≤ 15%.
5.4.1. Gb3
Total Gb3 was measured (total ion count (TIC)) for C16:0, C18:0, C20:0, C22:1, C22:0, C24:1, C24:0, and C24:OH isoforms simultaneously with creatinine in dried urine spots (DUS) as previously described [23,24]. Briefly, urine specimens were mixed carefully; then, 1 mL was deposited on a 5-cm-diameter disc of Whatman-GE 903 filter paper and dried completely at room temperature for at least 4 h. DUS extraction was performed by adding 4 mL of methanol to the filter paper disc and shaking for 1 h with an orbital shaker (300 rpm). Next, 10 µL of the extract was analyzed by high-performance liquid chromatography (HPLC) coupled to tandem mass spectrometry (MS/MS). The normal reference value was defined as ≤25 µg/mmol creatinine. Urine sample aliquots from an untreated Fabry male and an untreated Fabry female were used as high- and low-quality controls for this study. The intra- and interday precision values measured (RSD%) were respectively ≤12.5% and ≤11.3% for Gb3 and ≤10.1% and ≤7.0% for creatinine [24]. The intra- and interday accuracy values (Bias%) measured for Gb3 at a concentration of 3.75 µg/mL were respectively 4.3% and 7.9%.
5.4.2. Lyso-Gb3 and Related Analogues
Lyso-Gb3 and seven related analogues were analyzed by ultra-performance liquid chromatography (UPLC) coupled to MS/MS, following solid-phase extraction with Oasis mixed-mode strong cation exchange (MCX) cartridges (Waters Corp., Milford, MA, USA), as previously described [9,25]. Normal reference values were 0 pmol/mmol creatinine (not detected) for lyso-Gb3 and the analogues (−28 Da), (−12 Da), (−2 Da), and (+14 Da). Normal reference values were 17, 14, and 55 pmol/mmol creatinine for the lyso-Gb3 analogues (+16 Da), (+34 Da), and (+50 Da), respectively. Urine sample aliquots from an untreated Fabry male and an untreated Fabry female were used as high- and low-quality controls for this study. For lyso-Gb3 and its seven analogues, the intra- and interday assay precision values (RSD%) were respectively ≤13.0% and ≤20.1%. The intraday and interday accuracy values (Bias %) for lyso-Gb3 at concentrations of 1500, 11,000, and 24,000 pM were <7.9% [9].
5.5. Statistical Analyses
Statistical analyses were performed using IBM SPSS Statistics version 24, while bar charts were done using GraphPad Prism version 8.2.1. Paired-sample t-tests were performed to evaluate if the mean difference of the measured biomarker concentration was significantly different from zero depending on the collection time. Shapiro–Wilk’s test and visual inspection of a normal quantile–quantile (Q–Q) plot were used to determine if the differences in biomarker levels between the two collection times were normally distributed. If not, a mathematical transformation was applied to obtain normally distributed data. Levene’s test for equality of variances was performed to evaluate if the variance of the measured biomarker levels was equal for both collection times over 42 days.
For all analyses, statistical significance was established at p ≤ 0.05, and the Holm–Šídák procedure was used for multiple comparisons [26]. Specimen E, day 15 from patient 1 was excluded from statistics for lyso-Gb3 + analogue results only, considering that the lyso-Gb3 internal standard (IS) was off (−48%) for this specimen, leading to falsely elevated results. Specimen E, day 34 from patient 7 was also excluded from statistics, considering that it was an extreme outlier (>3 interquartile ranges from the 75th percentile). This might be related to a low creatinine value in this sample (1.8 mmol/L), leading to unreliable results.
Acknowledgments
We would like to thank Waters Corporation for their continued technical and scientific support.
Abbreviations
| CIUSSSE-CHUS | Centre Intégré Universitaire de Santé et de Services Sociaux de l’Estrie-Centre Hospitalier Universitaire de Sherbrooke |
| DUS | Dried urine spots |
| ERT | Enzyme replacement therapy |
| FD | Fabry disease |
| Ga2 | Galabiosylceramide |
| Gb3 | Globotriaosylceramide |
| HPLC | High-performance liquid chromatography |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| Lyso-Gb3 | Globotriaosylsphingosine |
| MCX | Mixed-mode strong cation exchange |
| MS/MS | Tandem mass spectrometry |
| REB | Research Ethics Board |
| RSD | Relative standard deviation |
| TIC | Total ion count |
| UPLC | Ultra-performance liquid chromatography |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/17/6114/s1, Table S1. Raw study dataset; ERT: enzyme replacement therapy.
Author Contributions
Conceptualization and design of the study, C.A.-B.; methodology, C.A.-B., P.L., M.B., M.-F.A., C.F., and C.M.; validation, C.A.-B., P.L., and M.B.; analysis of samples, M.A., A.T., M.E.-E., and I.M.; resources, C.A.-B., B.M., and D.G.B.; data curation, C.A.-B.; writing—original draft preparation, P.L., and M.B.; writing—review and editing, M.B., P.L., M.A., A.T., M.E.-E., I.M., M.-F.A., C.F., C.M., D.G.B., B.M., and C.A.-B.; supervision, C.A.-B.; project administration, C.A.-B., M.-F.A., C.F., C.M., and D.G.B.; funding acquisition, C.A.-B. All authors read and agreed to the published version of the manuscript.
Funding
This study was funded by personal research funds from C. Auray-Blais.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Juchniewicz P., Kloska A., Tylki-Szymanska A., Jakobkiewicz-Banecka J., Wegrzyn G., Moskot M., Gabig Ciminska M., Piotrowska E. Female fabry disease patients and X-chromosome inactivation. Gene. 2018;641:259–264. doi: 10.1016/j.gene.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 2.Clarke J.T.R. Narrative review: Fabry disease. Ann. Intern. Med. 2007;146:425–433. doi: 10.7326/0003-4819-146-6-200703200-00007. [DOI] [PubMed] [Google Scholar]
- 3.Michaud L., Auray-Blais C. Improved ways to screen for patients with fabry disease, involving optometry in a multidisciplinary approach. Can. J. Optom. 2012;74:25–32. doi: 10.15353/cjo.74.550. [DOI] [Google Scholar]
- 4.Lenders M., Stappers F., Niemietz C., Schmitz B., Boutin M., Ballmaier P.J., Zibert A., Schmidt H., Brand S.M., Auray-Blais C., et al. Mutation-specific fabry disease patient-derived cell model to evaluate the amenability to chaperone therapy. J. Med. Genet. 2019;56:548–556. doi: 10.1136/jmedgenet-2019-106005. [DOI] [PubMed] [Google Scholar]
- 5.Provençal P., Boutin M., Dworski S., Au B., Medin J.A., Auray-Blais C. Relative distribution of Gb3 isoforms/analogs in NOD/SCID/fabry mice tissues determined by tandem mass spectrometry. Bioanalysis. 2016;8:1793–1807. doi: 10.4155/bio-2016-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutin M., Menkovic I., Martineau T., Vaillancourt-Lavigueur V., Toupin A., Auray-Blais C. Separation and analysis of Lactosylceramide, Galabiosylceramide, and Globotriaosylceramide by LC-MS/MS in urine of fabry disease patients. Anal. Chem. 2017;89:13382–13390. doi: 10.1021/acs.analchem.7b03609. [DOI] [PubMed] [Google Scholar]
- 7.Abaoui M., Boutin M., Lavoie P., Auray-Blais C. tandem mass spectrometry multiplex analysis of methylated and non-methylated urinary Gb3 isoforms in fabry disease patients. Clin. Chim. Acta. 2016;452:191–198. doi: 10.1016/j.cca.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Boutin M., Auray-Blais C. Multiplex tandem mass spectrometry analysis of novel plasma lyso-Gb3-related analogues in fabry disease. Anal. Chem. 2014;86:3476–3483. doi: 10.1021/ac404000d. [DOI] [PubMed] [Google Scholar]
- 9.Lavoie P., Boutin M., Auray-Blais C. Multiplex analysis of novel urinary lyso-Gb3-related biomarkers for fabry disease by tandem mass spectrometry. Anal. Chem. 2013;85:1743–1752. doi: 10.1021/ac303033v. [DOI] [PubMed] [Google Scholar]
- 10.Auray-Blais C., Boutin M., Gagnon R., Dupont F.O., Lavoie P., Clarke J.T.R. Urinary globotriaosylsphingosine-related biomarkers for fabry disease targeted by metabolomics. Anal. Chem. 2012;84:2745–2753. doi: 10.1021/ac203433e. [DOI] [PubMed] [Google Scholar]
- 11.Dupont F.O., Gagnon R., Boutin M., Auray-Blais C. A Metabolomic study reveals novel plasma lyso-gb3 analogs as fabry disease biomarkers. Curr. Med. Chem. 2012;20:280–288. doi: 10.2174/0929867311320020008. [DOI] [PubMed] [Google Scholar]
- 12.Boutin M., Auray-Blais C. Metabolomic discovery of novel urinary Galabiosylceramide analogs as fabry disease biomarkers. J. Am. Soc. Mass Spectrom. 2015;26:499–510. doi: 10.1007/s13361-014-1060-3. [DOI] [PubMed] [Google Scholar]
- 13.Manwaring V., Boutin M., Auray-Blais C. A metabolomic study to identify new Globotriaosylceramide-related biomarkers in the plasma of fabry disease patients. Anal. Chem. 2013;85:9039–9048. doi: 10.1021/ac401542k. [DOI] [PubMed] [Google Scholar]
- 14.Auray-Blais C., Blais C.M., Ramaswami U., Boutin M., Germain D.P., Dyack S., Bodamer O., Pintos-Morell G., Clarke J.T., Bichet D.G., et al. Urinary biomarker investigation in children with fabry disease using tandem mass spectrometry. Clin. Chim. Acta. 2015;438:195–204. doi: 10.1016/j.cca.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Auray-Blais C., Lavoie P., Boutin M., Ntwari A., Hsu T.R., Huang C.K., Niu D.M. Biomarkers associated with clinical manifestations in fabry disease patients with a late-onset cardiac variant mutation. Clin. Chim. Acta. 2017;466:185–193. doi: 10.1016/j.cca.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Nowak A., Huynh-Do U., Krayenbuehl P.A., Beuschlein F., Schiffmann R., Barbey F. Fabry disease genotype, phenotype, and migalastat amenability: Insights from a national cohort. J. Inherit. Metab. Dis. 2020;43:326–333. doi: 10.1002/jimd.12167. [DOI] [PubMed] [Google Scholar]
- 17.Germain D.P., Elliott P.M., Falissard B., Fomin V.V., Hilz M.J., Jovanovic A., Kantola I., Linhart A., Mignani R., Namdar M., et al. The effect of enzyme replacement therapy on clinical outcomes in male patients with fabry disease: A systematic literature review by a European panel of experts. Mol. Genet. Metab. Rep. 2019;19:100454. doi: 10.1016/j.ymgmr.2019.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medin J.A. FACTS fabry gene therapy clinical trial: Two year data. Mol. Genet. Metab. 2019;126:S7–S16. doi: 10.1016/j.ymgme.2018.12.248. [DOI] [Google Scholar]
- 19.Wagner B.D., Accurso F.J., Laguna T.A. The applicability of urinary creatinine as a method of specimen normalization in the cystic fibrosis population. J. Cyst. Fibros. 2010;9:212–216. doi: 10.1016/j.jcf.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffmann R., Waldek S., Benigni A., Auray-Blais C. Biomarkers of fabry disease nephropathy. Clin. J. Am. Soc. Nephrol. 2010;5:360–364. doi: 10.2215/CJN.06090809. [DOI] [PubMed] [Google Scholar]
- 21.Mills K., Morris P., Lee P., Vellodi A., Waldek S., Young E., Winchester B. Measurement of urinary CDH and CTH by tandem mass spectrometry in patients hemizygous and heterozygous for Fabry disease. J. Inherit. Metab. Dis. 2005;28:35–48. doi: 10.1007/s10545-005-5263-4. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield P.D., Calvin J., Hogg S., O’Driscoll E., Halsall D., Burling K., Maguire G., Wright N., Cox T.M., Meikle P.J., et al. Monitoring enzyme replacement therapy in Fabry disease—Role of urine globotriaosylceramide. J. Inherit. Metab. Dis. 2005;28:21–33. doi: 10.1007/s10545-005-4415-x. [DOI] [PubMed] [Google Scholar]
- 23.Auray-Blais C., Lavoie P., Boutin M., Abaoui M. High-risk screening for fabry disease: Analysis by tandem mass spectrometry of Globotriaosylceramide (Gb3) in urine collected on filter paper. Curr. Protoc. Hum. Genet. 2017;93:17.26.1–17.26.12. doi: 10.1002/cphg.34. [DOI] [PubMed] [Google Scholar]
- 24.Auray-Blais C., Cyr D., Ntwari A., West M.L., Cox-Brinkman J., Bichet D.G., Germain D.P., Laframboise R., Melançon S.B., Stockley T., et al. Urinary Globotriaosylceramide excretion correlates with the genotype in children and adults with fabry disease. Mol. Genet. Metab. 2008;93:331–340. doi: 10.1016/j.ymgme.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Lavoie P., Boutin M., Abaoui M., Auray-Blais C. Fabry disease biomarkers: Analysis of urinary Lyso-Gb3 and seven related analogs using tandem mass spectrometry. Curr. Protoc. Hum. Genet. 2016;90:17.22.1–17.22.12. doi: 10.1002/cphg.1. [DOI] [PubMed] [Google Scholar]
- 26.Aickin M., Gensler H. Adjusting for multiple testing when reporting research results: The bonferroni vs holm methods. Am. J. Public Health. 1996;86:726–728. doi: 10.2105/AJPH.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.