Table 1.
Opioid and benzodiazepine receptor binding of 2′-fluorophenyl substituted imidazodiazepines.
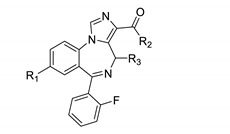
| Entry | Compound | R1 | R2 | R3 CH3 | KOR % 1 | MOR % 1 | DOR % 1 | BZR % 1 |
KOR (Ki, nM) |
BZR (Ki, nM) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GL-I-30 | HC≡C | t-BuO | (S) | 95 | 54 | 7 | 96 | 27 | 177 |
| 2 | GL-I-33 | HC≡C | t-PenO | (S) | 94 | 30 | 25 | 96 | 34 | 117 |
| 3 | GL-I-41 | HC≡C | t-Bu(H)N | (S) | 97 | 40 | 10 | 90 | 39 | 140 |
| 4 | GL-I-78 | c-Pr | EtO | (S) | 96 | 32 | 41 | 76 | 48 | 352 |
| 5 | SH-I-048B | Br | EtO | (S) | 95 | 0 | 0 | 61 | 63 | 96 |
| 6 | GL-I-32 | HC≡C | PrO | (S) | 95 | 9 | 14 | 98 | 64 | 148 |
| 7 | GL-I-31 | HC≡C | i-PrO | (S) | 94 | 32 | 8 | 97 | 65 | 245 |
| 8 | GL-I-38 | HC≡C | c-PrO | (S) | 95 | 18 | 16 | 96 | 68 | 127 |
| 9 | SH-I-047 | Br | EtO | (R) | 82 | 16 | 0 | 84 | 86 | 238 |
| 10 | SH-053-2′F-S-CH3 | HC≡C | EtO | (S) | 93 | 7 | 36 | 92 | 90 | 111 |
| 11 | GL-I-43 | HC≡C | Et(H)N | (S) | 95 | 22 | 3 | 94 | 102 | 44 |
| 12 | MP-III-023 | HC≡C | Me(H)N | (S) | 91 | 0 | 16 | 97 | 119 | 37 |
| 13 | MP-III-021 | HC≡C | MeO | (S) | 93 | 0 | 22 | 88 | 122 | 219 |
| 14 | GL-I-77 | HC≡C | EtS | (S) | 95 | 41 | 4 | 92 | 125 | 124 |
| 15 | GL-I-55 | HC≡C | c-Pr(H)N | (S) | 93 | 17 | 24 | 95 | 150 | 20 |
| 16 | GL-III-68 | c-Pr | Et(H)N | (R) | 88 | 0 | 10 | 100 | 150 | 452 |
| 17 | GL-III-42 | c-Pr | EtO | (R) | 86 | 0 | 13 | 62 | 174 | 726 |
| 18 | GL-II-74 | HC≡C | Et(H)N | (R) | 86 | 10 | 0 | 84 | 194 | 68 |
| 19 | GL-III-66 | HC≡C | i-Pr(H)N | (R) | 63 | 0 | 0 | 88 | 233 | 271 |
| 20 | MP-III-058 | Br | MeO | (R) | 84 | 0 | 4 | 86 | 237 | 290 |
| 21 | SH-053-2′F-R-CH3 | HC≡C | EtO | (R) | 89 | 28 | 32 | 85 | 240 | 379 |
| 22 | GL-II-75 | HC≡C | c-Pr(H)N | (R) | 81 | 0 | 3 | 85 | 278 | 93 |
| 23 | GL-II-76 | HC≡C | Pyrrolidine | (R) | 80 | 0 | 0 | 43 | 371 | - 2 |
| 24 | MP-III-022 | HC≡C | Me(H)N | (R) | 80 | 3 | 22 | 95 | 381 | 83 |
| 25 | GL-I-36 | HC≡C | F3CCH2O | (S) | 85 | 8 | 0 | 77 | 411 | 418 |
| 26 | GL-III-69 | Br | Me2N | (R) | 75 | 0 | 10 | 100 | 511 | 446 |
| 27 | MP-II-075 | HC≡C | BzO | H | 84 | 0 | 16 | 98 | 547 | 21 |
| 28 | MP-III-004 | HC≡C | MeO | (R) | 76 | 0 | 24 | 78 | 599 | 445 |
| 29 | GL-I-54 | HC≡C | Me2N | (S) | 78 | 18 | 11 | 94 | 788 | 90 |
| 30 | GL-III-70 | c-Pr | Me2N | (R) | 68 | 0 | 50 | 100 | 800 | 3395 |
| 31 | GL-II-73 | HC≡C | Me2N | (R) | 58 | 6 | 0 | 75 | 1189 | 506 |
| 32 | MP-III-019.B | HC≡C | H2N | (R) | 62 | 2 | 0 | 94 | 1534 | 54 |
| 33 | MP-III-018.B | HC≡C | H2N | (S) | 51 | 2 | 10 | 97 | 2782 | 17 |
| 34 | GL-III-54 | Cl | HO | (R) | 22 | 0 | 0 | 100 | - 2 | 42 |
| 35 | SH-053-2′F-S-CH3-Acid | HC≡C | HO | (S) | 20 | 0 | 18 | 93 | - 2 | 29 |
| 36 | SH-053-2′F-R-CH3-Acid | HC≡C | HO | (R) | 16 | 0 | 0 | 93 | - 2 | 37 |
| 37 | GL-II-93 | Br | HO | (R) | 0 | 0 | 0 | 73 | - 2 | 86 |
1 Percent inhibition at 10,000 nM; 2 dose response was carried out only for compounds with an inhibition of >50%.
