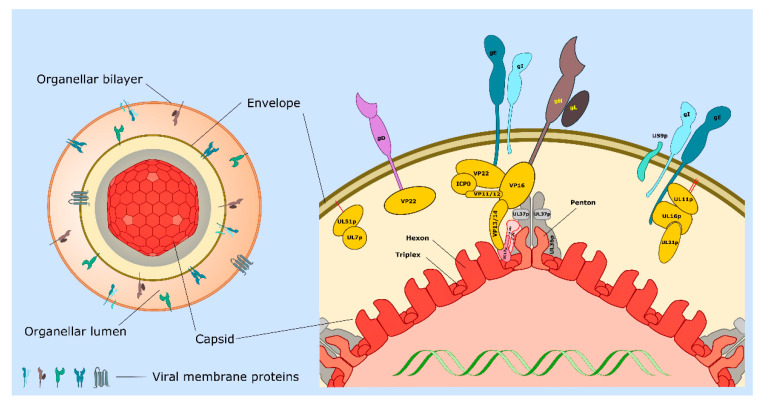
Figure 1.
Structure of the cytoplasmic herpes simplex virus type 1 (HSV-1) particle during egress. Left: The organelle-associated enveloped virion (OEV). The icosahedral capsid (with red hexons and pale red pentons) is bound to inner tegument proteins including UL36p and UL37p (gray layer), which provide the foundation for attachment of outer tegument (yellow). Tegument connects the capsid to the surrounding envelope (dark brown line) containing numerous single and multiple membrane-spanning envelope proteins. The mature virion resides within the lumen (orange) of the organelle utilized for envelopment. Prior to capsid envelopment the lipid bilayer of the bounding organelle must contain all of the membrane proteins that become incorporated into the mature envelope. This figure assumes that these envelope proteins persist in the bounding membrane of the OEV, though in most cases this is unknown. Right: Expanded view of a region of the capsid shell (red), inner- (gray), and outer- (yellow) tegument proteins and the envelope (dark brown bilayer). VP5 hexons and pentons are colored as in left-hand particle, and hexons, pentons, and triplexes are indicated. At the penton vertices each copy of VP5 is connected to a UL36p dimer and to adjacent triplexes via a UL17p/(UL25p)2 complex (for clarity the figure shows only two copies of VP5 at the vertex, and one copy of UL17p/(UL25p)2). Envelope proteins gD, gE/gI, gH/gL, and US9p (Table 1) are shown imbedded in the envelope bilayer such that their cytoplasmic tails project inward to connect with tegument proteins. Red lines indicate lipid anchors stabilizing the interaction of UL11p and UL51p with the envelope. Some proteins (for example gE and VP22) participate in multiple interactions within the tegument. These are drawn as separate complexes for clarity, but we do not mean to imply that these associations are necessarily exclusive of one another. See Table 1 and text for more details.