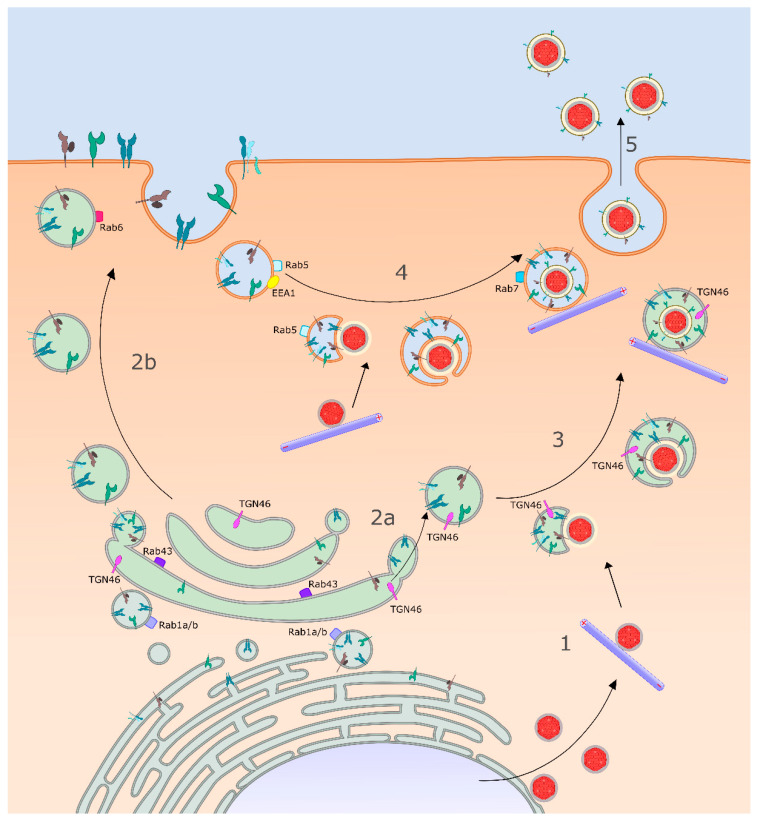
Figure 2.
HSV-1 cytoplasmic envelopment and trafficking. (1) HSV-1 capsids (red hexagons) emerge from the nucleus and inner tegument (gray layer) recruits kinesin for traffic to the plus ends of MTs (purple cylinders with + and - ends indicated) and to sites of cytoplasmic envelopment. Meanwhile, viral envelope proteins are processed via the conventional secretory pathway and traffic from the endoplasmic reticulum (ER) to the TGN (2a) and/or plasma membrane (2b). Rab1a/b (light purple squares) facilitate envelope protein export from the ER, while Rab43 (purple squares) supports transport through the Golgi apparatus and Rab6 (red square) is important for delivery of envelope proteins to the cell surface. (3) In one model HSV-1 capsids acquire their envelopes by attaching to, and budding into, the TGN or TGN-derived vesicles that contain the integral membrane protein TGN46 (violet lollipops). (4) Alternatively, cell-surface viral envelope proteins are endocytosed in a Rab5 (pale blue squares)-dependent manner into tubular–vesicular structures that provide the envelope. However, these tubular-vesicular structures lack the marker EEA1 (yellow oval) and thus are distinct from early endosomes. In other studies, egressing HSV-1 capsids have been observed to colocalize with early and late endosomes (Rab5- and Rab7-labeled, respectively). In all cases capsid envelopment is coupled to completion of the outer tegument (yellow layer around capsid) and incorporation of envelope proteins into the mature virion. (5) OEVs utilize MTs to deliver mature enveloped virions to the cell surface. See text for details.