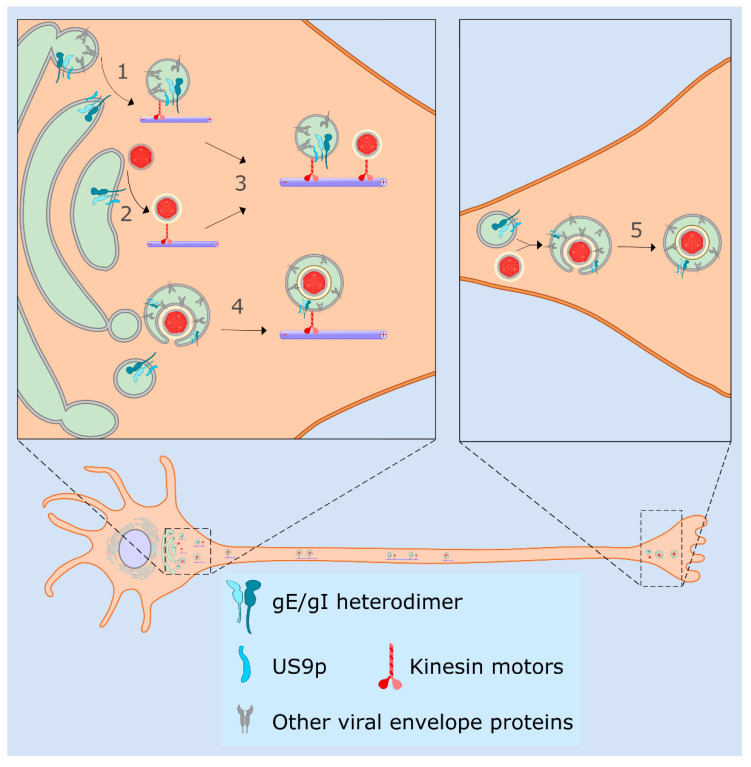
Figure 3.
Models for gE/gI-US9p-mediated sorting of HSV-1 during egress in neurons. In the “separate” model, transport vesicles carrying envelope protein cargo recruit kinesin motors using a gE/gI and/or US9p-dependent mechanism (1). Similarly, gE/gI and US9p “load” kinesin motors onto the surface of the HSV-1 capsid shell (2). Since gE/gI and US9p are imbedded in lipid bilayers this process is hypothesized to occur on the cytoplasmic face of an organelle (2), but the mechanism is not well understood. Capsids and transport vesicles then utilize these motors to traffic along cell body and axonal MTs (3) to the nerve terminal, and come together during capsid envelopment to generate the OEV (5). In the “married” model, HSV-1 capsids envelope at cytoplasmic organelles in the neuronal cell body (4) to generate the OEV, much as in non-neuronal cells (Figure 2). gE/gI and US9p may help drive this envelopment and/or subsequently function as membrane-bound receptors to recruit kinesins to the surface of the OEV. The OEV then traffics into and along the axon to transport its cargo of enveloped HSV-1 particles to the nerve terminal. The structures of gE/gI and US9p are represented at the bottom of the figure. Other viral and cellular components are as shown in Figure 1 and Figure 2. See text for more details.