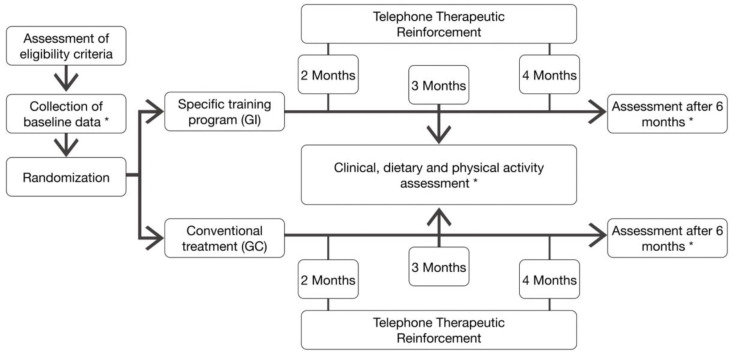
Figure 1.
Study Design. * Data collected at baseline and 6 months of intervention: (1) Anthropometric data (Body Mass Index, Neck circumference, Waist-to-hip ratio); (2) Physical activity (steps/day); (3) Questionnaires (Epworth Sleepiness Scale, Functional Outcomes of Sleep Questionnaire]; (4) Sleep-disordered breathing detected through polygraphy (Just for baseline and six months after intervention) (Apneas–Hypopneas Index, SpO2 mean (%), registered time spent with SpO2 < 90% (%) and Oxygen desaturation index); (5) Biochemical parameters (Urea, Creatinine, Alanine aminotransferase, Aspartate aminotransferase, Blood glucose, Total Cholesterol, High-density lipoprotein cholesterol, Low-density lipoprotein cholesterol and Triglycerides).