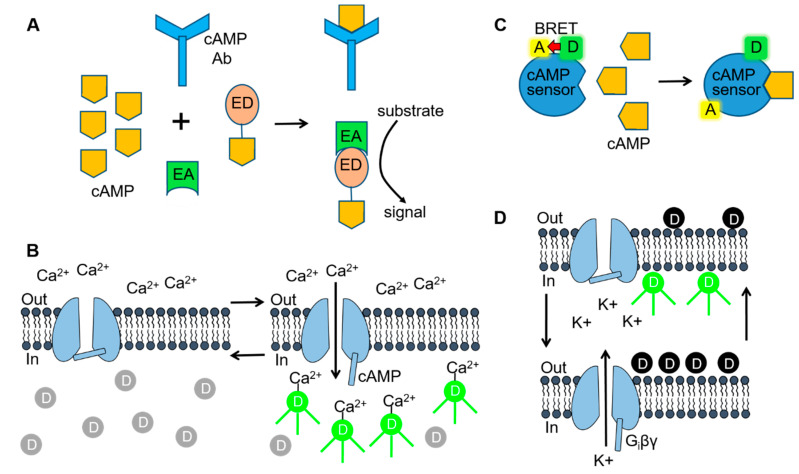
Figure 3.
Cell-based assay technologies used for delineating SC-mediated signaling. (A) Fragment complementation assays consist of inactive enzyme donor (ED) and enzyme acceptor (EA) components that form an active enzyme when combined. For measuring 3′,5′-adenosine monophosphate (cAMP), the ED consists of an inactive fragment of β-galactosidase that is conjugated to cAMP. When cellular cAMP levels are low or absent, the conjugated cAMP is sequestered by the cAMP antibody and no active enzyme is formed. In the presence of high levels of cAMP (as shown), the ED-cAMP conjugate is free to combine with the EA. β-galactosidase activity is then be detected by adding a substrate that is converted to a fluorescent or luminescent signal. (B) Cyclic nucleotide-gated (CNG) channel cAMP assay. Opening of CNG channels during elevations in intracellular cAMP allows Ca2+ to enter the cell and bind to Ca2+-sensitive fluorescent dye molecules. (C) Bioluminescence resonance energy transfer (BRET) assays use a biosensor consisting of a BRET donor (D) and acceptor (A) pair. The BRET cAMP sensor consists of a cAMP binding protein coupled to the BRET donor, Renilla luciferase (RLuc) and acceptor, yellow fluorescent protein (YFP). Binding of cAMP to the sensor (as shown) results in a conformational change and a loss of BRET intensity. (D) G protein-gated inward rectifier (GIRK) channel fluorescent membrane potential-sensitive dye (MPSD) assay. Hyperpolarization/depolarization of the cell resting membrane potential (left & right arrows), resulting from Gi protein βγ subunit (Giβγ) opening/closing of the GIRK channels, alters the distribution of MPSD molecules across the plasma membrane and thus the fluorescent signal. Figure 3A [70] was adapted with permission of Cambridge University Press through PLSclear. Figure 3C,D [71] were reproduced by permission from BMG Labtech and Taylor & Francis Ltd. (www.tandfonline.com), respectively.