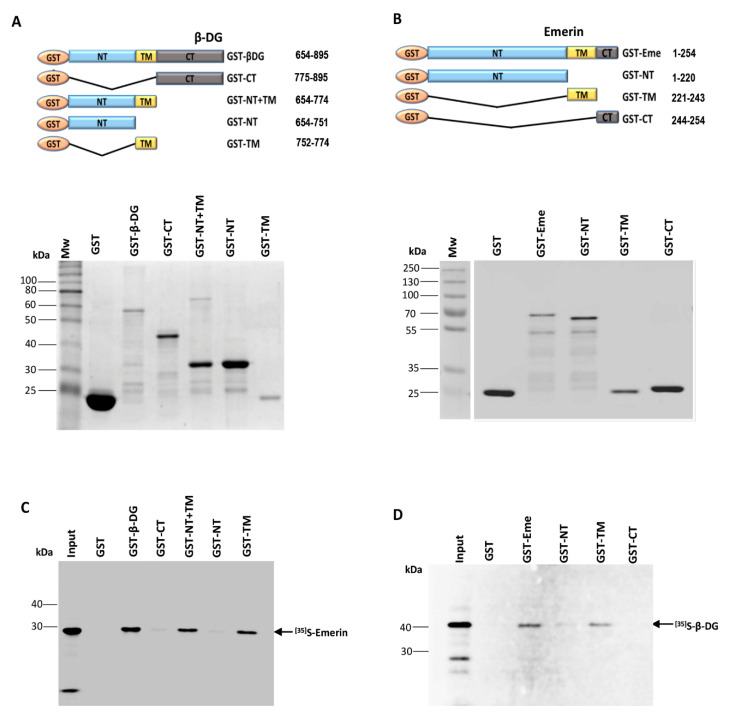
Figure 1.
Mapping of protein domains involved in the interaction between β-DG and emerin. (A) Top panel. Schematic representation of GST and GST-fusion proteins containing full-length β-DG or its separate domains, N-terminal domain (NT); C-terminal domain (CT), transmembrane domain (TM) and NT+CT domains. The numbers on the right indicate the amino acid residues of β-DG contained in each construct. Bottom panel. GST-tagged β-DG proteins were expressed in E. coli, purified using glutathione-Sepharose beads and visualized by SDS-PAGE followed by Coomassie blue staining. Mw, Protein molecular weight markers; (B) Top panel. Scheme showing GST and GST-fusion proteins containing full-length emerin or its separate domains, N-terminal (NT); C-terminal domain (CT) and transmembrane (TM) domains. The amino acid residues of emerin present in each construct are indicated on the right. Bottom panel. Representative Coomassie blue-stained gel showing the expression of GST and GST-tagged emerin proteins, expressed in bacteria and further purified as per (A). Mw, protein molecular weight markers; (C) GST and GST-tagged β-DG proteins immobilized on glutathione-Sepharose beads were incubated with in vitro labeled 35S-emerin to perform pull-down assays. Phosphorimaging results documenting interaction of 35S-emerin with GST–β-DG, GST–NT-TM and GST–TM, but not with GST–NT, GST-CT or GST alone are shown; (D) GST and GST-tagged emerin proteins previously immobilized on glutathione-Sepharose beads were incubated with in vitro translated 35S-β-DG to carry out in vitro interaction assays. Interaction of 35S-β-DG with GST–emerin and GST–TM, but not with GST–NT, GST-CT or GST alone was revealed by phosphorimaging analysis. (C,D) The input lanes correspond to 10% of the reticulocyte reaction used in the binding assays.