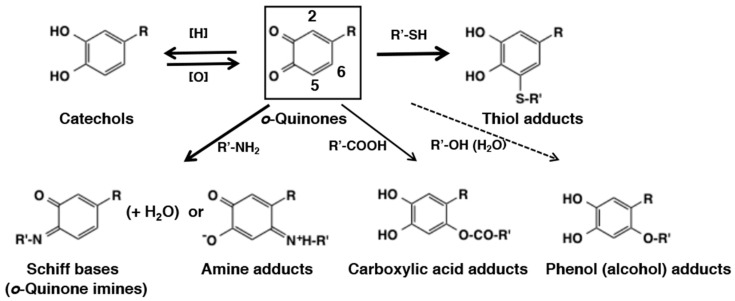
Figure 4.
Summary of intrinsic chemical reactivity of o-quinones. The relative reactivity is shown in the thickness of the arrows. Note that the reactions with carboxylic acids and alcohol (water) are possible only when the functional groups are present in the side chain of o-quinones. For the sake of simplicity, the numbering on the o-quinone ring is made so that R- is attached to the C1 position. The addition of thiols proceeds mostly at the C5 (major) and C2 (minor) positions (1,6-Michael addition) while the addition of other nucleophiles proceeds mostly at the C6 position (1,4-Michael addition).