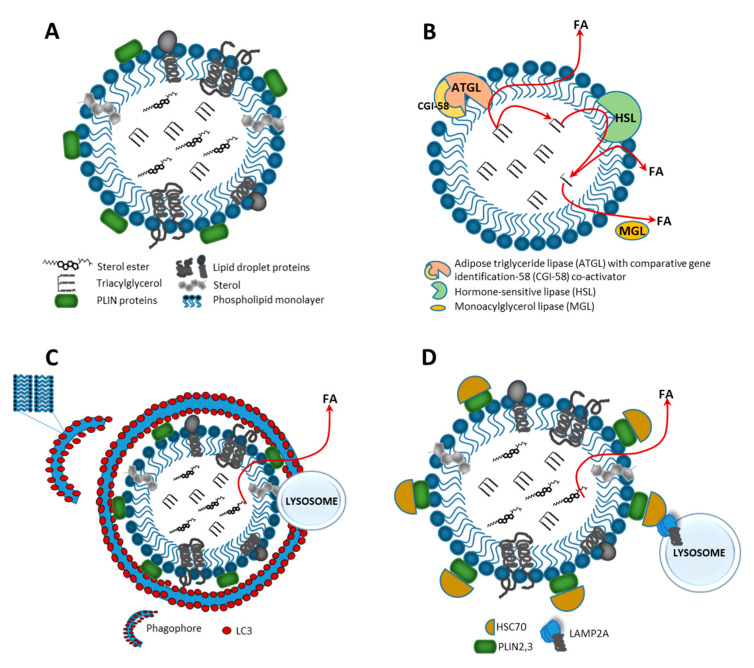
Figure 1.
Structure and catabolism of a lipid droplet (LD). (A) LD is surrounded by the phospholipid monolayer enclosing a core filled with neutral lipids, e.g., triacylglycerol (TAG) and sterol esters. Polar heads of phospholipids are oriented toward the cytosol, whereas their acyl chains contact the hydrophobic lipid core. The LD surface is associated with various proteins, e.g., members of the perilipin (PLIN) family. There are two major types of LD catabolism: lipolysis—an enzymatic hydrolysis of lipids in cytosol, and lipophagy—an autophagic/lysosomal pathway in the form of macroautophagy or chaperone-mediated autophagy (CMA). (B) In lipolysis, protein kinase A (PKA) phosphorylates PLIN1 proteins, leading to their proteasomal degradation and activating adipose triglyceride lipase (ATGL), which then initiates TAG hydrolysis to generate diacylglycerols (DAGs) and free fatty acids (FAs). Further degradation of DAGs occurs through activation of the hormone sensitive lipase (HSL), leading to monoacylglycerol (MAG) and FAs production. MAGs are released to the cytosol and cleaved by monoacylglycerol lipase (MGL) to generate glycerol and FAs. (C) In macroautophagy, the phagophore is formed and LC3 positive membranes engulf small LD or sequester portions of a large LD to form the autophagosome, which later fuses with lysosome where LD degradation and neutral lipid catabolism occur. (D) In chaperone-mediated autophagy, lipid droplet-coat proteins—PLIN2 and PLIN3—are degraded through a coordinated action of Hsc70 protein and lysosome-associated membrane protein 2A (LAMP2A) receptor; this makes the LD surface accessible to cytosolic lipases, which hydrolyze LD cargo to generate FAs, which next are released to the cytosol and undergo subsequent mitochondrial β-oxidation.