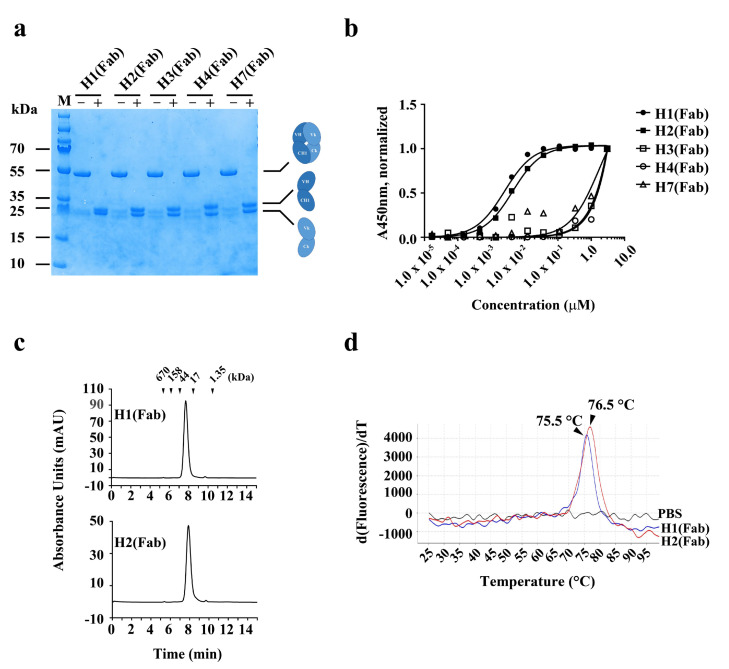
Figure 2.
Production and characterization of human anti-hYKL-40 Fabs. (a) SDS-PAGE analysis of two human anti-hYKL-40 Fabs, H1 (Fab) and H2 (Fab), purified from periplasmic extracts of E. coli transformed with the indicated expression vectors. + and − indicate with and without the reducing reagent (β-mercaptoethanol), respectively. (b) Soluble ELISA of serially diluted H1 (Fab) and H2 (Fab) on immobilized hYKL-40 surfaces to measure their apparent affinities (EC50, nM). (c) Size-exclusion chromatography analysis of H1 (Fab) and H2 (Fab). The positions of the molecular mass markers, shown as kDa, on the retention time x-axis are shown above the peaks. (d) Protein thermal shift assay of H1 (Fab) and H2 (Fab) to determine their thermal stability (Tm, °C). Fab: antigen-binding fragment; SDS-PAGE: sodium dodecyl sulfate-polyacrylamide gel electrophoresis; M: molecular mass marker.