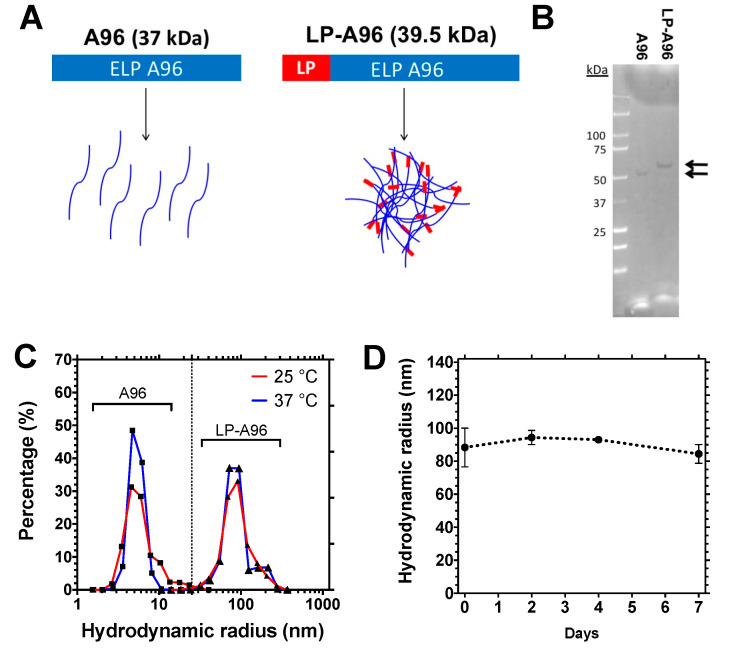
Figure 1.
Lacripep promotes assembly of LP-A96 into stable, multivalent spherical nanoparticles. (A) Design of LP-A96 fusion. Through heterologous expression in Escherichia coli, the LP was fused to the N-terminus of the elastin-like polypeptide (ELP), A96. (B) The identity and purity of A96 and LP-A96 were analyzed by Coomassie blue staining of samples resolved by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). Arrows indicate protein bands. A 20–30% upward shift in MW (molecular weight) with respect to the expected MW for ELPs and ELP fusions in SDS-PAGE has been observed by us and others [25,26]. The exact MW of LP-A96 was measured using MALDI-TOF-MS (Appendix A Figure A1D). (C) Comparison of the hydrodynamic radius of monomeric A96 and self-assembled LP-A96 nanoparticles at room and physiological temperatures. (D) The hydrodynamic radius of LP-A96 remains stable at 37 °C for least 7 days in DPBS (10 μM, n = 3, mean ± SD).