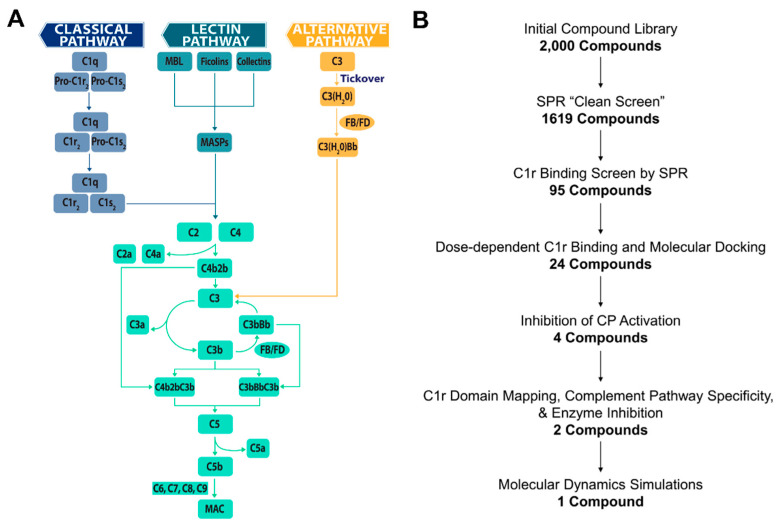
Figure 1.
(A) Complement is activated by three canonical pathways known as the classical pathway (CP), lectin pathway (LP), or alternative pathway (AP). Activation of the classical pathway is controlled by the C1 complex (i.e., C1qC1r2C1s2). The pattern recognition protein C1q binds to target surfaces resulting in the autoactivation of the zymogen C1r proteases (shown here as ‘Pro-C1r’) into C1r enzymes, which then proteolytically cleave and activate C1s within the C1 complex. The lectin pathway is activated by lectin pathway-specific pattern recognition proteins in complex with mannan-binding associated serine proteases (MASPs), while the alternative pathway is constitutively activated at low levels by a spontaneous hydrolytic event known as tick-over. Both the classical and lectin pathways converge at the cleavage of C2 and C4 to generate the classical/lectin pathway C3 convertases, C4b2b. Alternative pathway activation results in the formation of C3 convertases in the form of C3bBb. C3 convertases cleave the central molecule of the cascade, C3, into C3a and C3b, resulting in an amplification loop that produces increasing quantities of surface bound C3b. At high surface concentrations of C3b, C3 convertases bind an additional C3b molecule, resulting in a switch of substrate specificity to C5. Cleavage of C5 by these C5 convertases (i.e., C4b2bC3b and C3bBbC3b) results in the release of the anaphylatoxin C5a and the formation of the pore-like lytic structure called the membrane attack complex (i.e., C5b–C9). (B) Fragment-based drug discovery schematic.