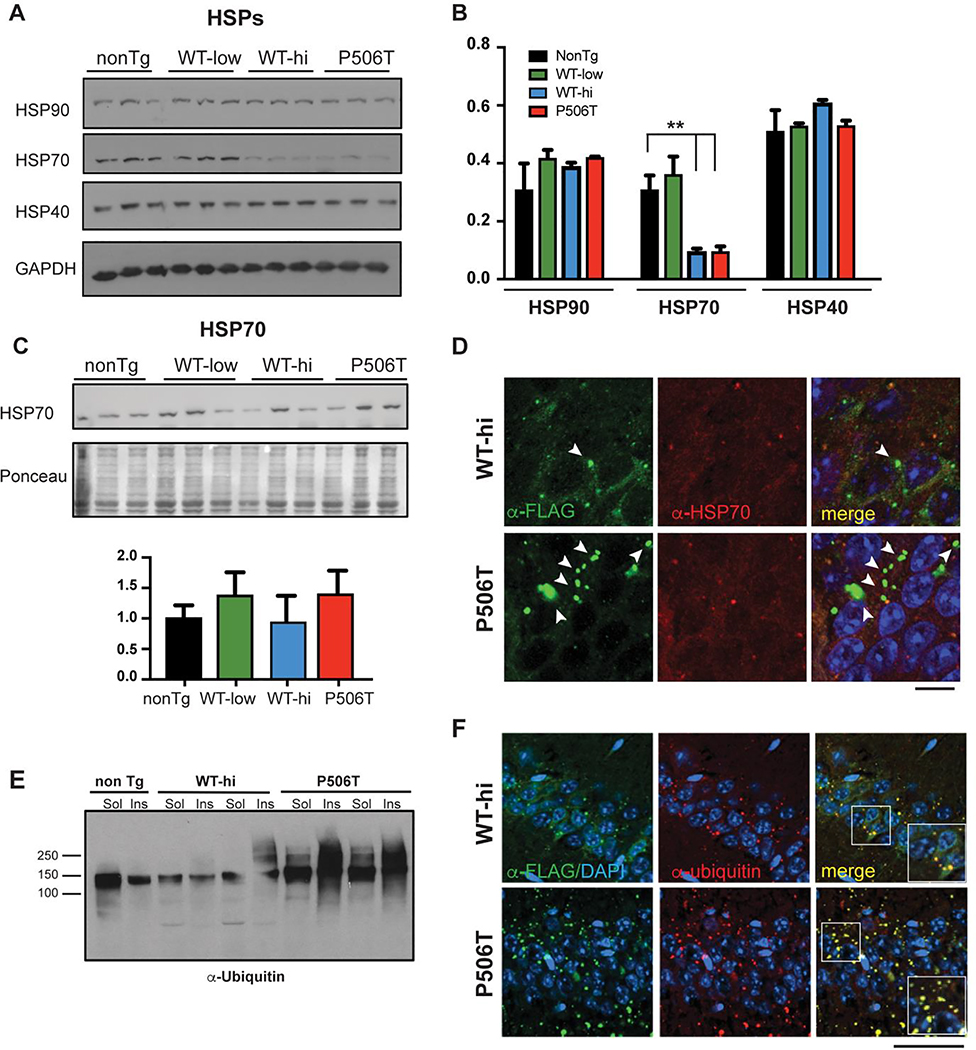
Figure 2. WT- and P506T-UBQLN2 induce changes in key protein quality control markers.
A, B. By Western blot analysis (A), HSP70 levels are significantly decreased in WT-hi and P506T mice while two other chaperones, HSP40 and HSP90, remain unchanged. Relative band intensities were quantified and graphed (B). ** = P< 0.01, error bars = ± SEM.
C. HSP70 levels are not elevated in the insoluble fraction of brain lysates from WT-hi and P506T mice on Western blot. Pellets from RIPA lysates of brain tissue were solubilized in 3X SDS sample buffer and analyzed by Western blot. Relative band intensities were quantified after normalization to Ponceau loading control and graphed.
D. Representative immunofluorescence tissue sections from the CA1 of WT-hi and P506T mice stained for transgenic FLAG-tagged UBQLN2 (green), HSP70 (red) and DAPI (blue). Arrows indicate occasional colocalization of HSP70 with small UBQLN2 positive puncta. Note that larger UBQLN2 aggregates (white arrows) are negative for HSP70 and occur more frequently in P506T mice. Scale bars: 10 μm.
E. Western blot of soluble and insoluble brain lysate fractions from transgenic and non-transgenic mice, probed with anti-ubiquitin antibody.
F. Co-immunofluorescence with antibodies detecting transgenic UBQLN2 (anti-FLAG, green) and ubiquitin (red) in the CA1 region of the hippocampus show that both UBQLN2 puncta (WT-hi mice) and aggregates (P506T mice) strongly co-localize with ubiquitin. Magnified (1.5x) insets in the merged panels highlight the colocalization of the staining (yellow). Scale bar: 50 μm.