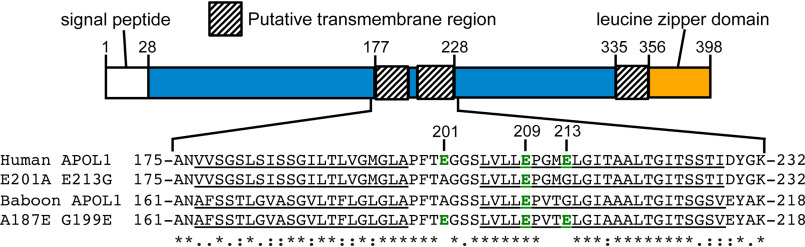
Figure 2.
Comparison of the human and baboon APOL1 H-L-H region. Top, schematic of full-length APOL1, showing human APOL1 residue positions of the signal peptide (white box, not present in recombinant protein) and putative transmembrane regions (shaded boxes). Regions of uncharacterized function are shown in blue. Bottom, amino acid sequence alignment of human APOL1, human APOL1 E201A/E213G, baboon APOL1, and baboon APOL1 A187E/G199E H-L-H regions. Delimiting amino acid residue numbers are shown directly above the schematic, whereas notable human-APOL1 residue positions are shown above the alignment. Note the presence of negatively charged residues (green type), within the otherwise uncharged, putative transmembrane helices (underlined). A baboon APOL1 deletion (N-terminal of the alignment) explains the disparate residue numbers of the baboon and human proteins. The alignment was used to identify fully conserved (*), strongly similar (:), or weakly similar (.) amino acid residues.