Figure 3.
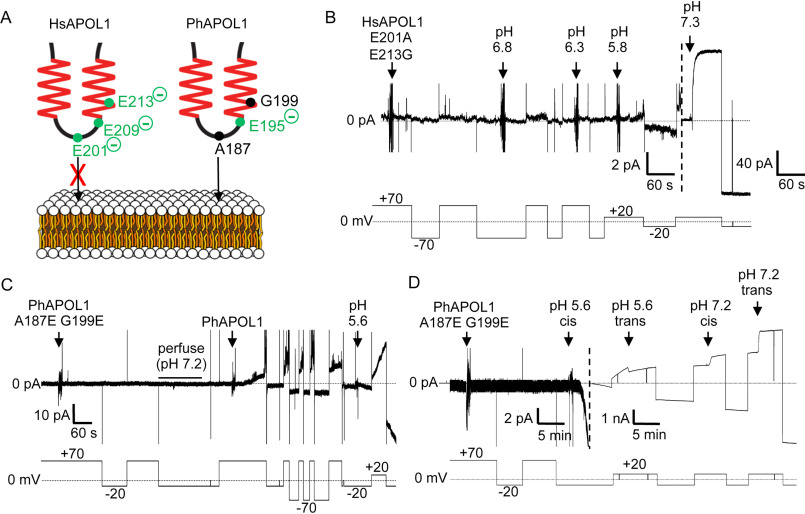
The H-L-H is involved in pH-dependent conductance formation by APOL1. A, schematic of human (left) and baboon (right) APOL1 H-L-H region showing human-specific glutamate residues (left), and their baboon-specific counterparts (right). At neutral pH, the three charged glutamates of the human H-L-H could impede lipid bilayer insertion of the human H-L-H to a greater extent than the baboon H-L-H, which has only one glutamate in this region (Glu-195). B–D, planar lipid bilayers were formed between symmetric solutions of chamber buffer (pH 7.2). Each panel represents a separate experiment, during which the experimenter set the voltage (bottom trace, mV) as the current was recorded (top trace, pA). B, a total of 500 ng of HsAPOL1 E201A/E213G was added to the cis side, which was then gradually acidified with HCl (2-pA scale, left side of dashed line). The current did not begin to change until after the pH was brought to 5.8. As expected, subsequent neutralization (pH 7.3) resulted in a large conductance increase due to channel opening (40-pA scale, right of dashed line). C, a total of 160 ng of PhAPOL1 A187E/G199E was added to the cis side, which caused no change in current. After perfusion with chamber buffer (pH 7.2) to remove soluble protein, the addition of just 80 ng of PhAPOL1 to the same bilayer resulted in a significant rate of current change at pH 7.2, which was then further increased by acidification to pH 5.6. D, 160 ng of PhAPOL1 A187E/G199E was added to the cis side, which again did not affect the current at pH 7.2; however, upon acidification to pH 5.6, a rapid increase in the current magnitude was observed, which was visible on the nA scale (right of dashed line). Similar to PhAPOL1 (see Fig. 4B), there was only a 3-fold increase in conductance upon subsequent pH neutralization.