Figure 5.
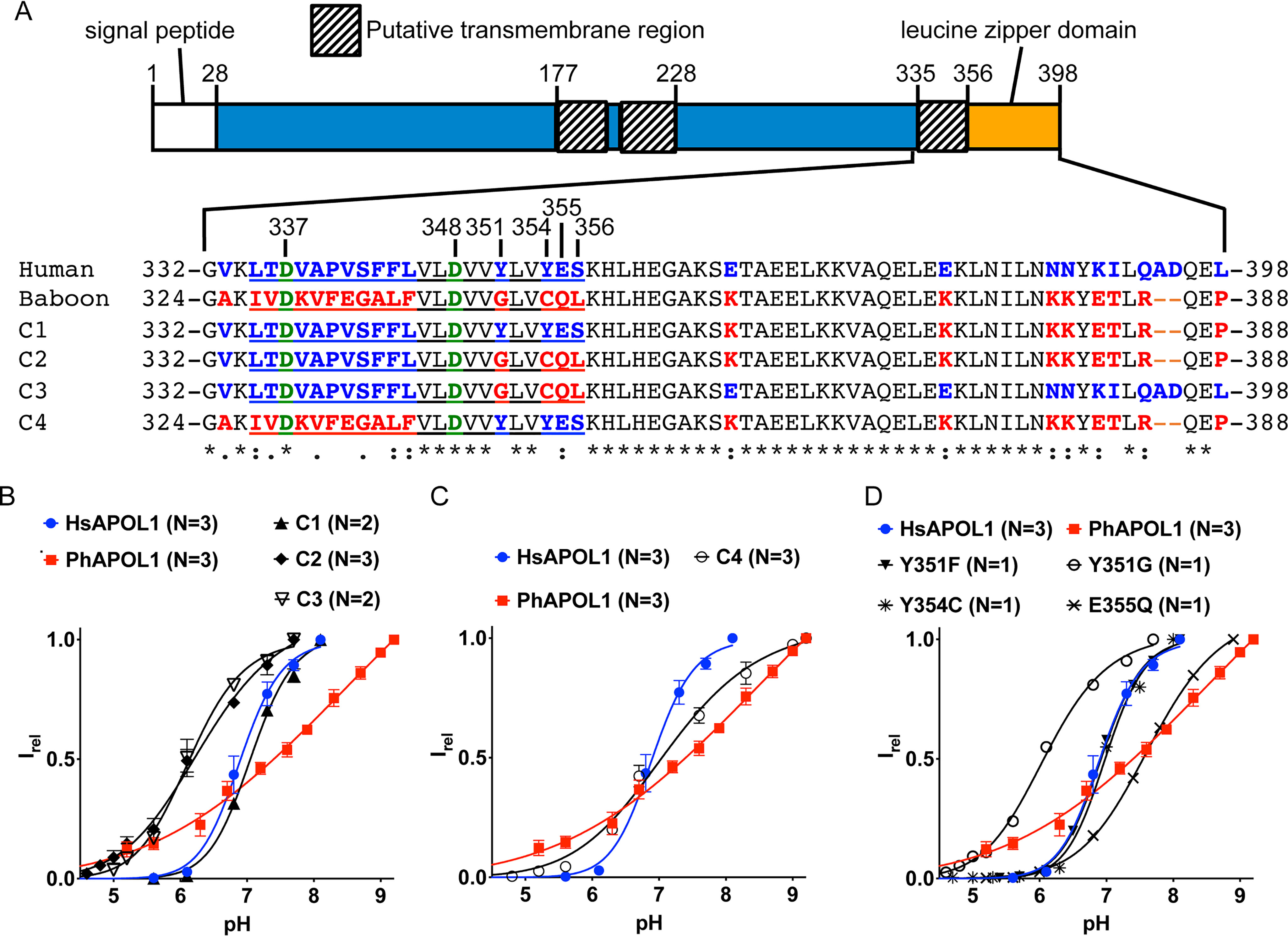
Substitution of CTD residues Tyr-351 and Glu-355 affects pH gating. A, amino acid alignment of human, baboon, and chimeric (C1–C4) APOL1 C-terminal domains, with relevant HsAPOL1 residue numbers indicated. A baboon APOL1 deletion (N-terminal of the alignment) explains the disparate residue numbers of the aligned amino acid sequences. The C1–C3 constructs differ from human APOL1 in that the indicated human-specific residues (blue type) were exchanged for their baboon-specific counterparts (red type). For example, C3 contains just four baboon-specific residues, and the remainder of the protein is identical to human APOL1, whereas C4 is the inverse chimera of C3; it differs from baboon APOL1 in that just four baboon-specific residues were substituted for their human-specific counterparts. Note that the four residues exchanged in C3 and C4 are situated at the C-terminal end of a putative transmembrane region (underlined). Situated in the putative transmembrane region are two conserved aspartate residues, Asp-337 and Asp-348 (green type; see “main text” for more details). A Clustal Omega alignment of the human and baboon sequences identified fully conserved (*), strongly similar (:), or weakly similar (.) amino acid residues. B–D, a conductance was obtained with each chimera, and pH titration experiments were performed as described in the legend to Fig. 4. The current at each pH value was normalized to the maximal current to obtain the relative current (Irel). Plotted is the average Irel of n independent experiments ± S.D. (error bars). The data were fit to the Hill equation. In some cases, error bars are smaller than the symbols used to represent data points.
