Figure 8.
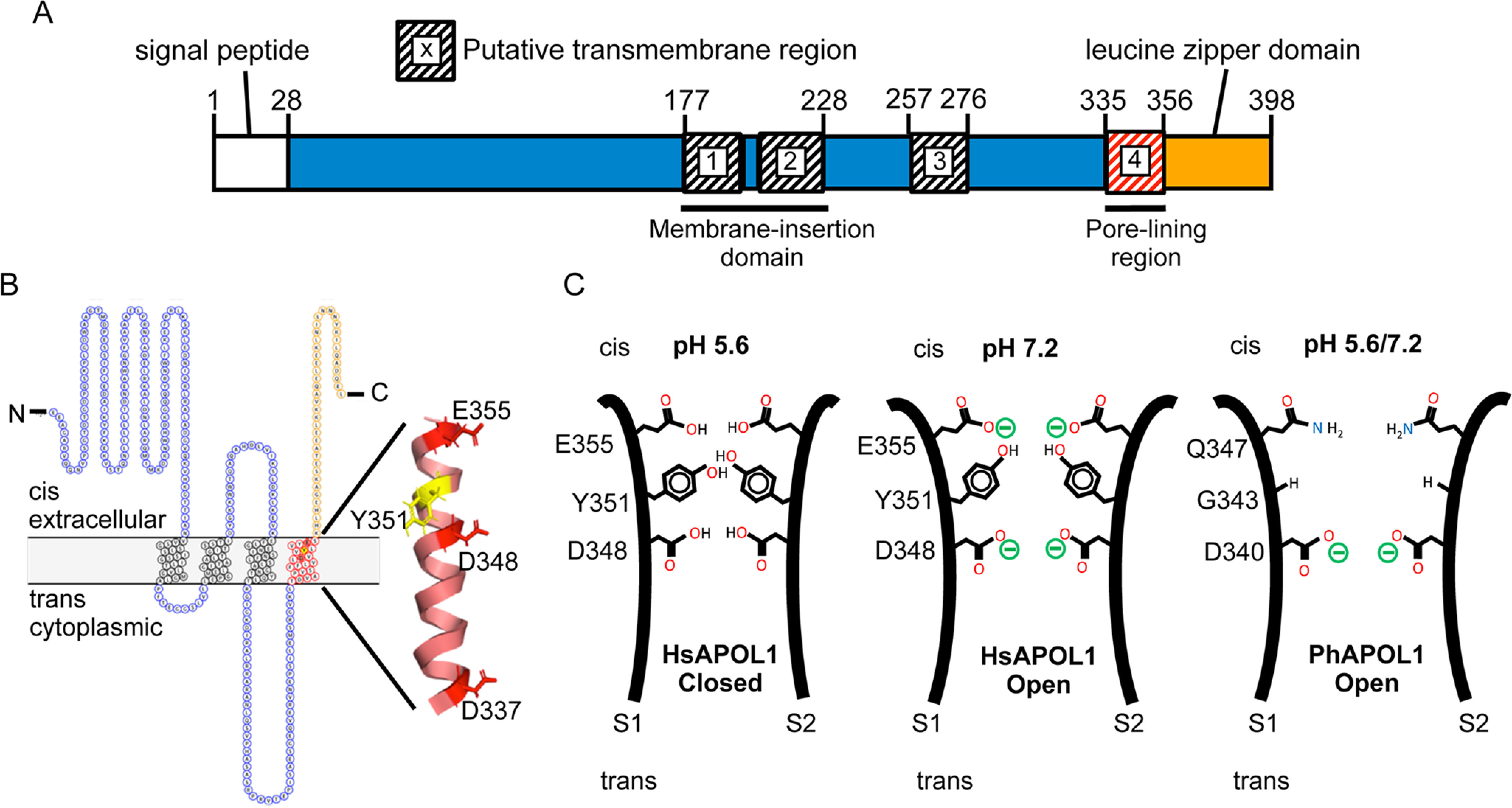
Modeling the domains and pore-lining region of the APOL1 channel. A, domain structure. The H-L-H encompasses a hairpin of two transmembrane helices (residues 177–228), which is likely responsible for membrane insertion. Our data suggest that a third transmembrane is situated between the H-L-H and the pore-lining region (residues 335–356), such that Glu-355 may be titrated by changes in cis pH. The leucine zipper domain (residues 357–398) may mediate interaction between APOL1 subunits and contains the binding site for the SRA protein of T. brucei rhodesiense. B, topology model. The cis side is equivalent to the extracellular side of the plasma membrane (42). The pore-lining region, shown in red (residues 335–356), is modeled as a transmembrane α-helix with the three acidic residues (Asp-337, Asp-348, and Glu-355) and tyrosine 351 aligned along the pore-lining face (43). C, speculative model of the pore. In this cross-section, the pore-lining region of two APOL1 subunits (S1 and S2) are brought into apposition via the C-terminal leucine zipper domain. The protonation status of Asp-348 determines pH gating and selectivity of the channel, with the Tyr-351 and Glu-355 pair having an accessory role in pH gating. Tyr-351 sterically blocks the channel in the closed state (pH 5.6), but channel opening is achieved by deprotonation of Glu-355 upon pH neutralization (pH 7.2). This exposes Asp-348 to the cis solution, allowing for its deprotonation and the conductance of cations through the channel. PhAPOL1 lacks Tyr-351 and Glu-355 equivalents (replaced by Gly-343 and Gln-347, respectively), and channel opening is less strictly dependent on pH.
