Reproductive failure is the hallmark of brucellosis in animals. An uncommon but important complication in pregnant women who become acutely infected with Brucella melitensis is spontaneous pregnancy loss or vertical transmission to the fetus. Unfortunately, the mechanism behind reproductive failure is still obscure, partially due to the lack of a proper study model. Recently, it was demonstrated that intratracheal (IT) inoculation of nonpregnant guinea pigs would replicate features of clinical disease in humans.
KEYWORDS: Brucella melitensis, intratracheal inoculation, aerosol inoculation, guinea pig, pregnant, vaccines
ABSTRACT
Reproductive failure is the hallmark of brucellosis in animals. An uncommon but important complication in pregnant women who become acutely infected with Brucella melitensis is spontaneous pregnancy loss or vertical transmission to the fetus. Unfortunately, the mechanism behind reproductive failure is still obscure, partially due to the lack of a proper study model. Recently, it was demonstrated that intratracheal (IT) inoculation of nonpregnant guinea pigs would replicate features of clinical disease in humans. To determine if IT inoculation would induce reproductive disease, guinea pigs were infected at mid-gestation and monitored daily for fever and abortions. Fever developed between day 14 to 18 postinoculation, and by 3 weeks postinoculation, 75% of pregnant guinea pigs experienced stillbirths or spontaneous abortions mimicking natural disease. Next, to investigate the guinea pig as a model for evaluating vaccine efficacy during pregnancy, nonpregnant guinea pigs were vaccinated with S19, 16MΔvjbR + Quil-A, or 100 μl PBS + Quil-A (as control). Guinea pigs were bred and vaccinated guinea pigs were challenged at mid-gestation with B. melitensis IT inoculation and monitored for fever and abortions. Vaccination with both vaccines prevented fever and protected against abortion. Together, this study indicates that pregnant guinea pigs are an appropriate animal model to study reproductive disease and offer an improved model to evaluate the ability of vaccine candidates to protect against a serious manifestation of disease.
INTRODUCTION
Brucellosis is one of the most commonly reported zoonotic diseases, with a worldwide distribution (1). Of the 12 recognized species, Brucella melitensis is considered the most virulent and is associated with the majority of human cases (2). In its natural hosts of sheep and goats, B. melitensis infection results in spontaneous mid-gestational abortion and placentitis (3). Disease transmission to humans occurs after ingestion of unpasteurized dairy products or exposure to infectious aerosols (3). The acute illness manifests with nonspecific flu-like symptoms, including undulant fever, malaise, and anorexia. Alarmingly, recent epidemiological evidence also indicates that reproductive disease occurs in women who become infected during pregnancy and can result in first or second term spontaneous pregnancy loss or transmission to the fetus (4–7).
The pathogenesis of reproductive brucellosis in natural host species, as well as in humans, is a subject of considerable interest. Reproductive disease during pregnancy has been investigated in natural host (small ruminants, cattle, and suids) as well as in laboratory animal models (mice, guinea pigs, nonhuman primates) (8, 9). Utilizing the natural hosts to study Brucella pathogenesis presents numerous challenges, as it requires biosafety level 3-agriculture facilities (BSL-3Ag) and is more expensive and time-consuming due to the large size of the animals and greater length of gestation. As an alternative, mice are commonly utilized for studying host-pathogen interactions and for investigating vaccine candidates (10). The mouse model has outpaced the use of other animal models, such as guinea pigs or nonhuman primates, due to the ease of housing large numbers of animals and the ready availability of reagents for evaluating the immune response to infection. The mouse presents several limitations as a model for human reproductive disease though, such as a difference in placentation and failure to abort regardless of dose or timing of inoculation with Brucella spp., instead exhibiting fetal resorptions when infected at day 4.5 of gestation (11, 12).
Guinea pigs were used extensively in the past for pathogenesis investigations and to develop and evaluate vaccines for Brucella spp. (13). As a reproductive model, advantages to the guinea pig include similar placentation to humans and a relatively longer length of gestation (∼65 days). Furthermore, a study found that when pregnant guinea pigs were inoculated via intramuscular (IM) injection at mid-gestation with 1 × 105 CFU B. abortus 544, they experienced stillbirths and spontaneous abortions (14). While these results are intriguing, IM inoculation represents an artificial route of exposure for brucellosis. Intratracheal (IT) inoculation simulates aerosol exposure and is a more natural route of infection. Using IT inoculation, we have previously demonstrated that nonpregnant guinea pigs develop fever and systemic disease when inoculated with B. melitensis (15). In this study, we built upon this foundation by using intratracheal inoculation of pregnant guinea pigs to determine the effect upon reproductive success and to evaluate the pregnant guinea pig as an improved animal model for vaccine efficacy and safety.
RESULTS
Intratracheal inoculation results in systemic infection.
A previous study demonstrated that nonpregnant guinea pigs will develop fever, splenomegaly, and systemic colonization following IT inoculation with 1 × 107 CFU B. melitensis (15). However, the response of the pregnant guinea pig to infection with Brucella spp. has not been well established. To determine if IT inoculation of pregnant guinea pigs would result in similar clinical signs and systemic colonization, guinea pigs at mid-gestation (∼30 to 35 days) were inoculated with either 50 μl of 1 × 107 CFU B. melitensis 16M or 50 μl of phosphate-buffered saline (PBS). Mid-gestation was selected as the optimal time point to inoculate as, similar to natural hosts, infection during this period induces the highest rate of abortions and stillbirths (16). Following inoculation, guinea pigs were euthanized at weekly intervals to determine the kinetics of infection during pregnancy.
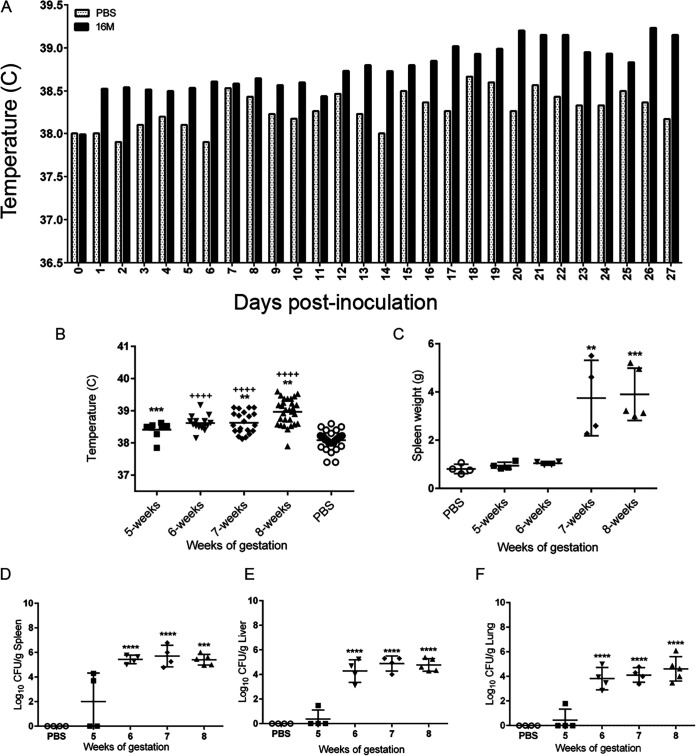
An important clinical marker of brucellosis in humans is fever. Nonpregnant female guinea pigs develop fever, defined as a temperature of ≥39.5°C, approximately 14 to 18 days following IT inoculation with 1 × 107 CFU (15, 17). As expected, the mean body temperature was increased in pregnant guinea pigs inoculated with B. melitensis compared to those receiving PBS (Fig. 1A). The mean temperature of the guinea pigs inoculated with B. melitensis was statistically increased at 8 weeks of gestation compared to PBS controls and to guinea pigs inoculated at 5 and 7 weeks of gestation (w.g.) (Fig. 1B). Development of fever in the pregnant guinea pig correlates with the time frame of fever development in nonpregnant guinea pigs and in human cases (15, 18).
FIG 1.
Brucella infection results in a higher mean temperature, splenomegaly, and systemic colonization. (A) Kinetics of temperature following intratracheal inoculation with 1 × 107 CFU B. melitensis 16M. Implantable subcutaneous microchips were monitored daily using a handheld DAS-7000 reader. Mean daily temperature was increased in the guinea pigs inoculated with B. melitensis compared to PBS controls. (B) Mean temperature was compared between groups using ANOVA followed by Dunnett’s multiple comparisons. ++++, P < 0.0001 compared to 8 weeks of gestion. **, P < 0.01; ***, P < 0.001, compared to PBS controls. (C) Spleen weight was compared between groups using ANOVA. **, P < 0.01; ***, P < 0.001 compared to PBS controls. Bacterial colonization from pregnant guinea pigs at 5, 6, 7, and 8 weeks of gestation following intratracheal inoculation with 1 × 107 CFU B. melitensis. Spleen (D), liver (E), and lung (F) tissues were collected at each time point for bacterial culture on Farrell’s medium. The recovery of organisms is plotted as the total CFU/g (mean ± standard deviation [SD]). Mean recovery per gram of tissue was compared between time points and uninfected control guinea pigs. Statistical significance was determined by ANOVA followed by Dunnett’s multiple comparisons. ***, P < 0.001; ****, P < 0.0001.
Splenomegaly or spleen enlargement is another common finding in human and animal cases of brucellosis. Furthermore, nonpregnant guinea pigs develop splenic enlargement following IT inoculation. The spleens of pregnant guinea pigs were weighed at the time of euthanasia to determine if infection affected weight. Spleen weight was not affected by inoculation at 5 or 6 w.g. However, infection with B. melitensis significantly increased spleen weight at 7 to 8 w.g., as seen in nonpregnant guinea pigs (P < 0.001; Fig. 1C).
Brucella spp. have a well-defined tropism for reticuloendothelial organs, such as the spleen, liver, and lung, in both experimental animal models and natural hosts. To determine if IT inoculation resulted in colonization of systemic organs, spleen, liver, and lung tissues were evaluated by culture on Farrell’s medium. By 1 week postinoculation (p.i.) and 5 w.g., 3 of 4 pregnant guinea pigs (75%) had colonization of at least one of these tissues (Fig. 1D to F). By 2 weeks p.i./6 w.g., colonization of the spleen, liver, and lung was detected in 100% of the pregnant guinea pigs (n = 4), and mean colonization was statistically increased (P < 0.0001) from 2 to 4 weeks p.i./6 to 8 w.g. (Fig. 1D to F). This pattern was similar to that seen in nonpregnant female guinea pigs in which bacteria colonized systemic organs by 2 weeks p.i. and persisted for 4 weeks p.i. (15).
To corroborate colonization findings, spleen, liver, and lung samples were collected for histopathology. Slides were graded in a blinded fashion for inflammation type and severity as previously described (Table S1 in the supplemental material) (15). By 1 week p.i., histopathologic changes in the lung were confined to mild pulmonary edema (Fig. S2). Lung lesions from week 6 of gestation to week 8 consisted of an embolic pattern of aggregates of neutrophils and macrophages in the alveoli and bronchioles, which suggests the guinea pigs developed bacteremia. Histopathologic findings in the spleen were similar to those seen in nonpregnant female guinea pigs and included lymphoid hyperplasia during the early stages of infection that progressed to foci of macrophages by 3 and 4 weeks p.i./7 to 8 w.g. (Fig. S2). The most significant pathology in the liver occurred in the 3 and 4 weeks p.i./7 to 8 w.g. groups and included lesions commonly associated with brucellosis in people, such as microgranulomas, hepatic necrosis, and lymphocytic periportal inflammation (Fig. S2). Taken together, these results indicate that pregnant guinea pigs develop histopathological changes typically observed in natural cases of Brucella infection.
Infection of pregnant guinea pigs with B. melitensis results in spontaneous abortion, placental colonization and inflammation, and vertical transmission.
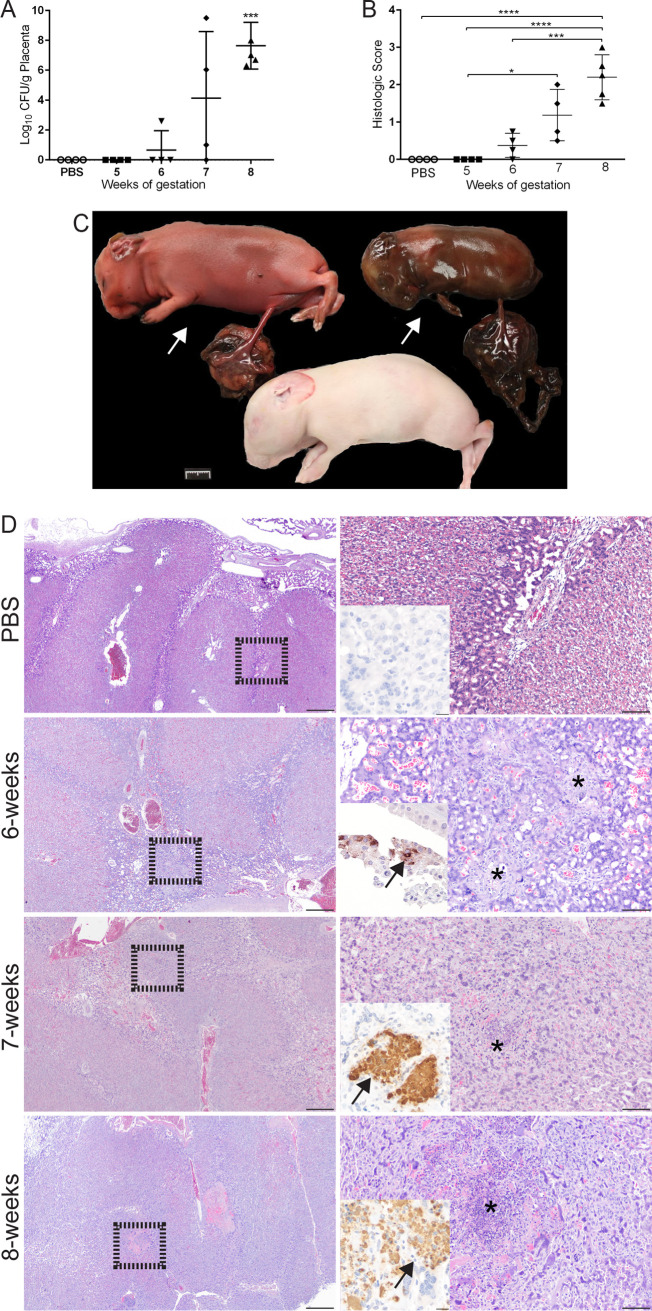
Reproductive disease is a common sequela of infection with B. melitensis during pregnancy in natural hosts, but adverse pregnancy events may also occur in pregnant women. In order to study the pathogenesis of reproductive disease, an animal model is needed that has similar placentation and replicates key features of the disease, such as abortion and fever. To determine if pregnant guinea pigs would offer an improved animal model for reproductive brucellosis, guinea pigs (n = 4) at mid-gestation (∼4 weeks) were infected with 50 μl of 1 × 107 CFU B. melitensis 16M or 50 μl PBS via intratracheal inoculation. Guinea pigs were then monitored twice daily for adverse pregnancy events, defined as hemorrhagic vaginal discharge or abortions. Since infection can also result in stillborn offspring, crown to rump (C-R) length was measured to estimate the stage of gestation for each fetus to determine if in utero fetal death had occurred. No abortions or stillborn offspring were noted in the negative controls or at 5 or 6 w.g. (Table 1). However, by 7 w.g., 1 of 4 pregnant guinea pigs (25%) had bloody vaginal discharge on day 21 p.i., and 3 of 17 (17.6%) offspring were stillborn (Fig. 2C). Within the 8 w.g. cohort, 1 of 5 (20%) guinea pigs spontaneously aborted at 24 days p.i. and, in total, 6 of 20 (30%) offspring were stillborn (Table 1). These results indicate that IT inoculation of pregnant guinea pigs with B. melitensis generates adverse obstetric outcomes.
TABLE 1.
Crown to rump length, number of offspring, fetal viability, and colonization of fetuses from pregnant guinea pigs infected with 1 × 107 CFU B. melitensis ITa
| Wk of gestation | Avg. C-R length (cm) | No. of offspring | No. stillborn (%) | Spleen (avg. log10/g) | Liver (avg. log10/g) | Lung (avg. log10/g) | Stomach contents (no. [%]) |
|---|---|---|---|---|---|---|---|
| Wk 5 | 40.8 | 24 | 0/24 (0) | 0 | 0 | 0 | 0/24 (0) |
| Wk 6 | 46.8 | 21 | 0/21 (0) | 0.02 | 0.14 | 0.13 | 0/21 (0) |
| Wk 7 | 59.9 | 17 | 3/17 (17.6) | 0.93 | 1.13 | 0.93 | 6/17 (35.3) |
| Wk 8 | 50.8 | 20 | 6/20 (30) | 0.57 | 0.86 | 0.40 | 2/20 (10) |
C-R length was measured from the base of the skull to the point of the hip and is reported as the average (avg.) length in centimeters (cm). Fetal viability is reported as the number (no.) and percentage born stillborn. Fetal spleen, liver, lung, and stomach contents were cultured on Farrell’s medium and results are reported as the avg. log10/g. Maternal infection variably resulted in vertical transmission to the fetus. Results of culture of stomach contents are reported as number and percentage with growth.
FIG 2.
Level of placental colonization correlates with clinical signs and severity of histologic lesions. (A) A representative placenta sample from each pregnant guinea pig (n = 4) per time point was cultured on Farrell’s medium. The recovery of organisms is plotted as the total CFU/g (mean ± SD). Mean recovery per gram of tissue was compared between time points and uninfected control guinea pigs. Statistical significance was determined by ANOVA followed by Dunnett’s multiple comparisons. *, P < 0.05; ***, P < 0.001. (B) Histologic scores from placenta. H&E stained sections of placenta were graded in a blinded fashion for edema (0 to 1), mononuclear infiltrate (0 to 4), necrosis (0 to 4), and bacteria (0 to 1). Values represent the mean histological score per group (n = 4). Values that are significantly different are indicated by bars and asterisks. ***, P < 0.001, ****, P < 0.0001. (C) Representative gross image of discolored and desiccated stillborn fetuses (white arrows) with inappropriate C-R length and necrosis of the subplacenta from a pregnant guinea pig 3 weeks postinfection. Note the apparently normal fetus from the same pregnancy. Marker = 1 cm. (D) Representative sections of H&E-stained and IHC-labeled placenta at the time of abortion or study endpoints. Note the abundant intracytoplasmic staining in the chorionic epithelium at 6 weeks of gestation (arrow), indicating maternal infection precedes fetal infection. By 7 weeks of gestation, multifocal areas of necrosis (asterisk) with abundant intracytoplasmic Brucella antigen scattered throughout the labyrinth of the placenta (inset, arrow). Lesions become more numerous and larger by 8 weeks of gestation (asterisk). Left column H&E, magnification 2×; bar = 500 μm. Dashed box indicates field selected for higher magnification in the right column. Right column H&E, magnification 10×; bar = 50 μm. Inset, IHC magnification 40×; bar = 25 μm.
To determine if placental and uterine colonization correlated with the development of clinical signs, placenta and uterus were cultured on Farrell’s medium. No colonization was noted in the PBS group or at 5 w.g., and only 1 pregnant guinea pig in the 6 w.g. group had a low level of colonization (2.6 logs) of the placenta (Fig. 2A), which correlated with the lack of clinical symptoms seen in these groups. However, by 6 w.g., 3 of 4 (75%) pregnant guinea pigs had colonization of the placenta, but the level of colonization was highly variable, with a range of 1 to 9.5 logs. By 8 w.g., placenta of 4 of 4 (100%) pregnant guinea pigs were colonized. These data suggest that it takes approximately 3 weeks in the pregnant guinea pig to establish an infection severe enough to result in spontaneous abortion and stillborn offspring.
In the natural host, Brucella species infection results in colonization of the placenta and also generates a severe necrotizing lesion that precedes reproductive failure. To further characterize the effect of infection on the placenta in the pregnant guinea pigs, samples were graded on a histologic scale (0 to 4) for type and degree of inflammation, necrosis, and edema (Table S1) and were stained with a polyclonal Brucella-specific antibody to confirm Brucella antigen within areas of inflammation. Beginning at 6 w.g., small foci of neutrophils and histiocytes were scattered throughout the labyrinth layer of the placenta, which is the site of fetal-maternal blood exchange (19). Immunohistochemistry (IHC) revealed intracellular antigen within the chorioallantoic epithelium (Fig. 2D), suggesting the earliest infection may be of the cytotrophoblasts, which are derived from the maternal side of the placenta (20). Inflammation became more severe and widely distributed by 7 and 8 w.g., and the character of the lesion shifted from small infiltrates to multifocal aggregates of histiocytes and foci of necrosis in the labyrinth. IHC with a Brucella-specific antibody confirmed the etiology of these lesions, which demonstrated abundant intracellular antigen within inflammatory foci centered on the site of fetal/maternal blood exchange (Fig. 2D). Since necrotizing placentitis is the most common histologic placental lesion in ruminant cases of brucellosis, these results indicate that the pregnant guinea pig replicates a key feature of disease.
Finally, to characterize the effect of maternal infection on fetal colonization defined as vertical transmission, tissues (spleen, liver, lung, and stomach contents) were collected from each fetus. Fetuses in the negative-control groups and at 5 w.g. had appropriate C-R length, indicating that early fetal death did not occur, and no bacteria were recovered from any tissue (Table 1) (19). Even though all of the fetuses had appropriate C-R length in the 6 w.g. group, colonization of the liver and lung was detected in 4 of 17 (23.5%), indicating that bacteria are capable of crossing the placental barrier and infecting the fetuses by 2 weeks p.i./6 w.g. (Table 1). Interestingly, although only 17.6% of fetuses appeared stillborn by 3 weeks p.i., colonization occurred in 11 of 17 fetuses (64.7%), suggesting that infection of the fetus does not necessarily result in fetal death. Vertical transmission occurred in 6 of 20 (30%) fetuses from pregnant guinea pigs at 8 w.g. Tissue culture revealed that lung is the best tissue for confirming colonization of the fetus and further indicates that pregnant guinea pigs develop disease with similar clinical manifestations as natural hosts and humans.
Intratracheal inoculation results in colonization and inflammation of the mammary gland.
Colonization of the mammary gland is an important means of transmission of disease from infected ruminants to humans because Brucella spp. are shed in the milk (16, 21). It is unknown if this aspect of infection would be replicated in a pregnant guinea pig model, so left and right mammary gland were collected for culture on Farrell’s medium following IT inoculation with 1 × 107 CFU/50 μl B. melitensis 16M. Histopathology and IHC were performed to correlate colonization with microscopic evidence of infection.
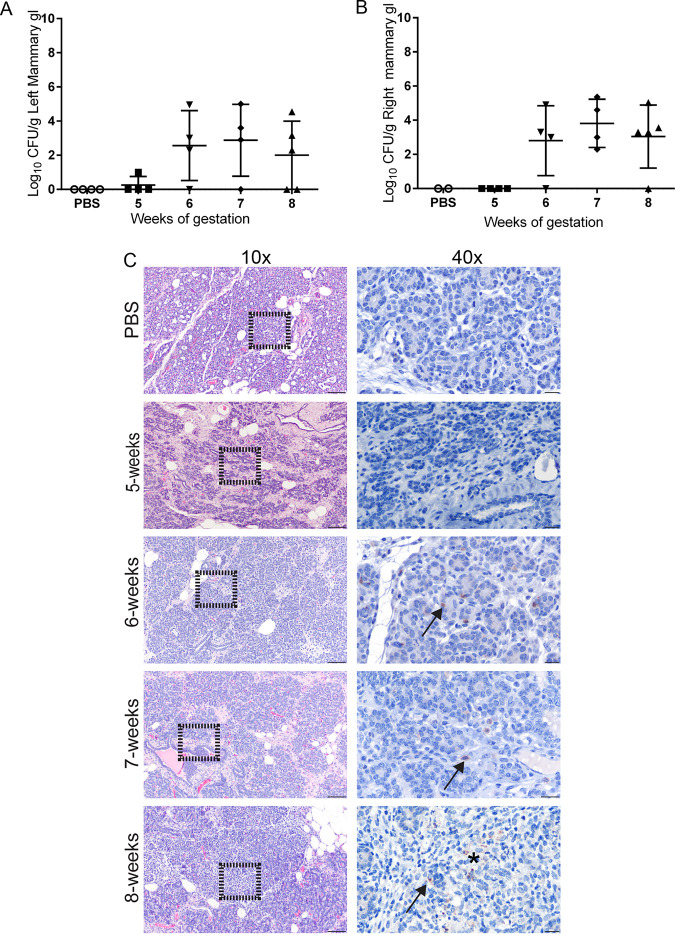
A low level of colonization (1 log) of the mammary gland was detected at 1 week p.i. in 1 of 4 pregnant guinea pigs (25%) (Fig. 3A and B), which was associated with a minimal infiltrate of neutrophils (Fig. 3C). No antigen was detected by IHC at 5 w.g. By 6 w.g., colonization was detected in 4 of 4 (100%) pregnant guinea pigs in at least one half of the mammary gland. Histologically, this was accompanied by a mild to moderate interstitial infiltrate of neutrophils and macrophages at 6 w.g., and Brucella antigen was detected by IHC within the cytoplasm of macrophages in the interstitium and in glandular epithelial cells (Fig. 3C). By 3 weeks p.i., the inflammatory population had shifted toward one reflective of chronic inflammation, including macrophages, lymphocytes, and plasma cells. Again, Brucella antigen was detected within both macrophages and glandular epithelium (Fig. 3C). At 8 w.g., aggregates of macrophages were multifocally scattered throughout the interstitium and replaced mammary acini (Fig. 3C). These results indicate that intratracheal inoculation results in disseminated infection and could lead to shedding in the milk. These data further support that the pregnant guinea pig is an appropriate animal model to study all aspects of Brucella-induced disease.
FIG 3.
Colonization of the mammary gland results in inflammation. Colonization of the left (A) and right (B) mammary gland following IT inoculation with 1 × 107 CFU B. melitensis. The recovery of organisms is plotted as the total CFU/g (mean ± SD). Mean recovery per gram of tissue was compared between time points and uninfected control guinea pigs. Differences between time points and negative controls are not statistically significant. (C) Representative H&E and IHC images of mammary gland at each time point postinoculation. Left column, H&E, magnification 10×; bar = 50 μm. Right column, higher magnification of area delineated by black outline at 10×. Multifocal macrophages and epithelial cells contain granular intracellular (arrows) and extracellular (*) Brucella antigen. IHC with polyclonal anti-Brucella antibody, magnification 40×; bar = 25 μm.
Infection with B. melitensis stimulates a Brucella-specific IgG humoral response.
Humans and animals infected with Brucella spp. will develop a Brucella-specific antibody response. Previous data indicated that nonpregnant guinea pigs develop an IgG response to challenge with B. melitensis. Indirect enyzme-linked immunosorbent assay (iELISA) was used to evaluate the kinetics of Brucella-specific IgG with guinea pig sera at 0, 7, 14, 21, and 28 days p.i. Infection with Brucella melitensis resulted in a statistically significant increase in IgG levels beginning at 6 w.g. (P < 0.0001) and continuing through 8 w.g. (P < 0.0001) (Fig. S3).
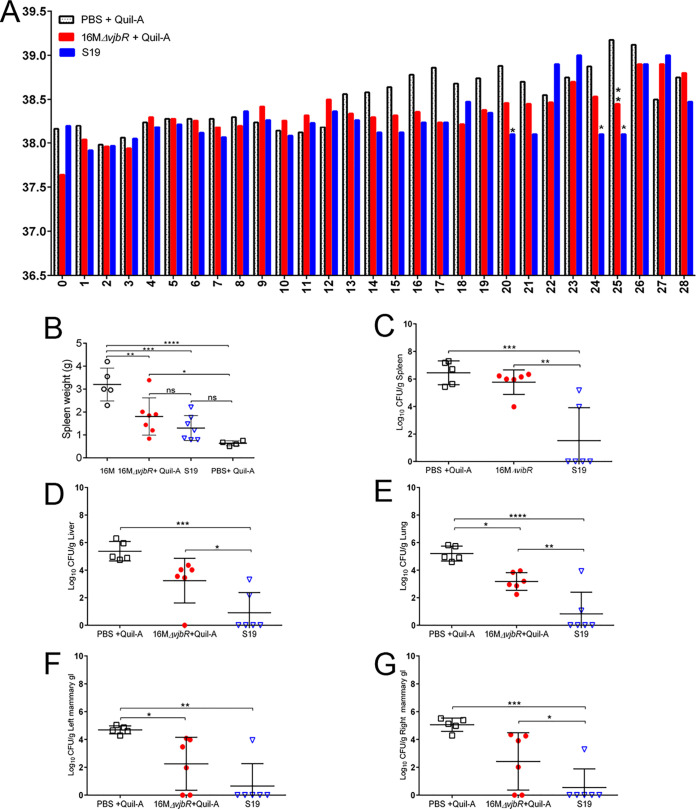
Vaccination with S19 and 16MΔvjbR + Quil-A prevented fever and adverse pregnancy events.
After demonstrating that pregnant guinea pigs develop clinical disease when infected with B. melitensis via IT inoculation, the pregnant guinea pig was next evaluated as an animal model for vaccine safety and efficacy. To do so, the protocol depicted in Fig. S1 and described in the Materials and Methods section was developed. Nonpregnant guinea pigs were vaccinated with 1 × 109 CFU/100 μl S19 to simulate the vaccination dose and schedule used in domestic animals, in which nonpregnant female cattle are vaccinated with 3 × 108 to 5 × 109 S19 organisms subcutaneously (16). B. abortus S19 is widely used to vaccinate cattle against brucellosis and is the reference strain by which other vaccines are measured (16). 16MΔvjbR has been extensively evaluated by our laboratory in the mouse model, where it has proven safe and efficacious (12, 22). However, the vaccine has not been evaluated in pregnant mice, and therefore efficacy in the pregnant guinea pig was evaluated to further demonstrate the utility of the pregnant guinea pig as a model.
Pregnancy was confirmed by abdominal palpation, and pregnant guinea pigs at approximately 35 days of gestation (range: 35 to 40 days) were challenged via IT inoculation with 1 × 107 CFU/50 μl B. melitensis 16M (19). The estimated gestation range at the time of challenge was calculated by measuring the C-R length of the offspring, comparing it to an established chart of C-R length for fetal guinea pigs, and subtracting from 62, which is the average length of gestation for the guinea pig (19). Even though guinea pigs were vaccinated with B. abortus S19, animals were challenged with B. melitensis because the effects of this strain on pregnancy had been established and previous studies have demonstrated cross-protection among strains in guinea pigs and cattle (23, 24). Clinically, protection was defined as absence of fever and adverse pregnancy events, including stillbirths or abortions.
As expected, pregnant guinea pigs sham vaccinated with PBS + Quil-A had a higher mean daily temperature than vaccinated guinea pigs (Fig. 4A). Intriguingly, none of the pregnant guinea pigs in the groups vaccinated with S19 or the 16MΔvjbR + 10 μg Quil-A developed fever, and they had a lower mean daily temperature than 16M-inoculated guinea pigs, indicating vaccination protected against one of the key clinical features of disease (Fig. 4A).
FIG 4.
Vaccination prevented fever and reduced colonization of the systemic tissues. (A) Vaccination with 16MΔvjbR and S19 decreased the mean daily temperature following IT challenge with 1 × 107 CFU B. melitensis. The numbers along the x axis indicate days postchallenge. (B) To evaluate splenomegaly, spleen tissue was collected at necropsy and weighed. Mean spleen weight was decreased in vaccinated groups compared to 16M-challenged guinea pigs. Spleen (C), liver (D), lung (E), left mammary gland (F), and right mammary gland (G) tissues were assessed for colonization by culturing on Farrell’s medium and plotted as the total CFU/g (mean ± SD). Statistical significance was determined by ANOVA followed by Tukey’s multiple comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Vaccine efficacy was evaluated by determining if vaccination prevented or reduced colonization in tissues. Spleen, liver, lung, and left and right mammary gland tissues were collected at the time of parturition or 28 days p.i. to evaluate colonization and histopathologic changes. As the kinetics study indicated pregnant guinea pigs would develop colonization within 28 days p.i., animals in the vaccine efficacy study were euthanized at this time point to evaluate protection against systemic infection. Spleen weight was also assessed because it was expected that vaccination would reduce splenomegaly following challenge with 16M.
Spleen weight was significantly decreased in guinea pigs previously vaccinated with S19 (P < 0.001) and 16MΔvjbR (P < 0.01) compared to unvaccinated controls following challenge with wild type (WT) (Fig. 4B). When colonization of organs was evaluated following challenge, guinea pigs that were previously vaccinated with S19 had a statistically significant reduction in mean colonization (Fig. 4C to E) of the spleen (P < 0.001), liver (P < 0.001), and lung (P < 0.0001). In 4 of 6 (66.7%) animals, S19 vaccination reduced colonization to below the limit of detection (10 CFU/g tissue) following challenge. Vaccination with 16MΔvjbR did not result in a significant decrease in colonization in the majority of tissues compared to unvaccinated controls, with the exception that vaccination decreased colonization of the lung (P < 0.05) (Fig. 4E).
When the spleen was examined by light microscopy, S19-vaccinated animals had mild lymphoid hyperplasia, indicating immune stimulation but not active inflammation. However, the spleen of 16MΔvjbR-vaccinated guinea pigs had microscopic lesions which resembled those seen in unvaccinated controls, suggesting vaccination did not prevent a low level of infection from occurring after challenge with WT. When a histologic grading scale was applied, the 16MΔvjbR-vaccinated group had an intermediate degree of inflammation (mean = 0.86) compared to S19 vaccinated (mean = 0.2) and unvaccinated controls (mean = 1.39).
After demonstrating that pregnant guinea pigs develop colonization of the mammary tissues following IT inoculation with B. melitensis, efficacy was evaluated by determining if vaccination would prevent or decrease colonization following challenge. S19 resulted in a significant decrease in mean colonization of the left mammary gland (P < 0.01) and right mammary gland (P < 0.001). Guinea pigs vaccinated with 16MΔvjbR had a more modest decrease in colonization of the left mammary gland (P < 0.05) and no significant difference in the right (Fig. 4F and G).
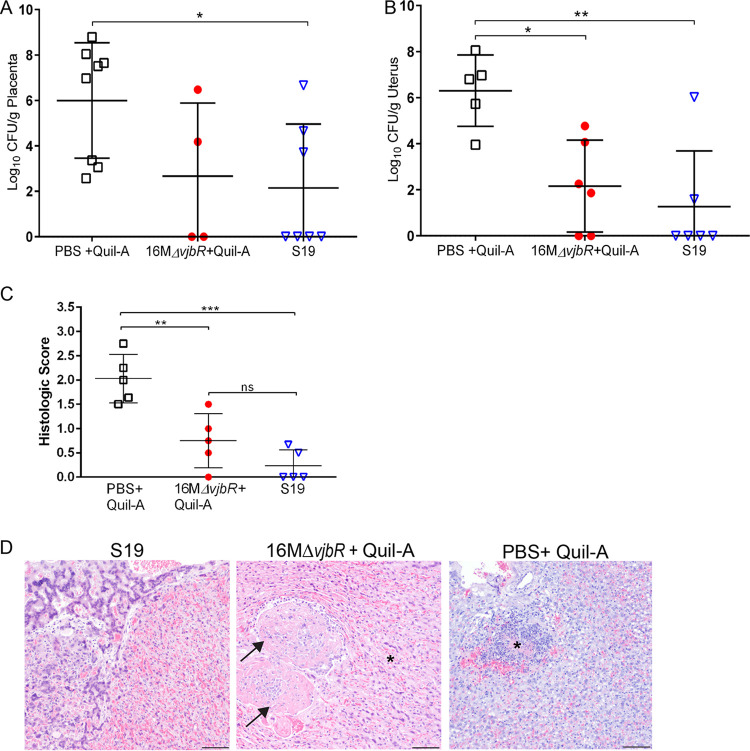
Adverse pregnancy events such as abortion and stillbirths secondary to infection with B. melitensis are a common disease manifestation, and a primary goal of vaccination is to prevent the development of reproductive disease (3, 18). As expected, spontaneous abortion and/or stillbirths were noted in three of the unvaccinated guinea pigs (60%), but neither vaccinated group had abortions or stillbirths. Adverse pregnancy events were associated with a mean colonization of 5.99 logs in the unvaccinated guinea pigs (Fig. 5A). Even though the vaccines protected against the development of clinical signs, neither vaccine was 100% effective at preventing colonization of the uterus and placenta. However, S19 resulted in statistically significant reduction in mean colonization of the uterus (P < 0.01) and placenta (P < 0.05) compared to unvaccinated guinea pigs following challenge (Fig. 5A and B). As noted previously, colonization of the placenta predisposes the animal to pregnancy loss. Thus, vaccination with S19 and 16MΔvjbR likely prevented the development of placental insufficiency that leads to pregnancy loss. When the placenta was evaluated by light microscopy and a histologic grading scale was applied, S19-vaccinated animals had a lower mean histologic score (0.24) than 16MΔvjbR-vaccinated (0.55) or unvaccinated (1.82) animals (Fig. 5C). S19-vaccinated guinea pigs had rare infiltrates of macrophages within the labyrinth, but 16MΔvjbR-vaccinated and unvaccinated guinea pigs had more extensive areas of inflammation following challenge with WT (Fig. 5D).
FIG 5.
Vaccination reduces colonization and inflammation of the placenta following challenge with B. melitensis. Colonization of the placenta (A) and uterus (B) in pregnant guinea pigs previously vaccinated with either 1 × 109 CFU/100 μl Brucella abortus S19 (n = 6), Brucella melitensis 16MΔvjbR + 10 μg Quil-A (n = 6), or sham vaccinated with 100 μl sterile PBS + 10 μg Quil-A (n = 5). Twelve weeks postvaccination and at mid-gestation (∼35 to 40 days of gestation), pregnant guinea pigs were challenged by IT inoculation with 1 × 107 CFU B. melitensis. The recovery of organisms is plotted as the total CFU/g (mean ± SD). Mean recovery per gram of tissue was compared between vaccinated and unvaccinated guinea pigs using ANOVA followed by Dunnett’s multiple comparisons. *, P < 0.05; **, P < 0.01. (C) H&E-stained sections of placenta were graded in a blinded fashion for edema (0 to 1), mononuclear infiltrate (0 to 4), necrosis (0 to 4), and bacteria (0 to 1). Values represent the mean histological score per group (n = 5). Values that are significantly different are indicated by bars and asterisks. **, P < 0.01; ***, P < 0.001; ns, not significant. (D) Representative images of H&E-stained placenta from each group. No lesions were noted in the S19-vaccinated guinea pigs. Areas of coagulative necrosis (asterisk) were noted adjacent to thrombi (arrow) in 16MΔvjbR + Quil-A-vaccinated guinea pigs. PBS + Quil-A-inoculated guinea pigs developed foci of necrosis (asterisk) following challenge with 16M. Magnification 10×; bar = 50 μm.
To monitor the immune response to vaccination, the Brucella-specific IgG antibody response was measured by analyzing serum samples from prevaccination to 16 weeks postvaccination. Both S19 and 16MΔvjbR vaccination resulted in a statistically significant (P < 0.001) IgG response as early as 4 weeks postvaccination (Fig. S4). In response to challenge with 1 × 107 CFU B. melitensis, the memory response in both vaccines was rapid and statistically increased compared to unvaccinated animals that were challenged. Interestingly, even though S19 resulted in protection below the limit of detection in 66.7% of pregnant guinea pigs, the IgG response was significantly less than that provided by 16MΔvjbR, indicating IgG response is inadequate to predict protection.
DISCUSSION
Reproductive disease is an important consequence of infection with Brucella spp., but the pathogenesis is not fully understood. Infection in pregnant guinea pigs was evaluated because guinea pigs have similar placentation to humans and have been used successfully to model other bacterial reproductive pathogens such as Treponema pallidum (syphilis), Chlamydia trachomatis, and Listeria monocytogenes (25). Despite the apparent relevance of the pregnant guinea pig, the pregnant mouse model is more commonly utilized to investigate events underlying reproductive failure. When pregnant mice are inoculated at day 4.5 of gestation with WT or live attenuated strains (LAV), fetal death and resorption will occur (11, 26, 27). However, the mouse model does have some limitations, including differences in placentation, degree of trophoblast invasion, and a short gestation that may make it less suitable for fully exploring the reproductive pathogenesis of Brucella spp. (28). This study demonstrates that pregnant guinea pigs are a more physiologically relevant model because they develop fever, abortions, and stillbirths when infected with B. melitensis, as seen in humans and natural hosts. Importantly, because 75% to 80% of pregnant guinea pigs develop fever in response to infection, body temperature can be used as an indicator of vaccine safety and efficacy in future studies.
Infectious aerosols are a known transmission route for brucellae in both humans and animals, so IT inoculation was evaluated in the pregnant guinea pig to determine if reproductive disease would occur (2). IT inoculation is a more physiologically relevant route of infection than intraperitoneal (i.p.) challenge, and a previous study demonstrated that it reliably results in clinical signs and systemic disease in guinea pigs (15). Pregnant guinea pigs have rarely been used in brucellosis research, with only one previous study evaluating the pregnant guinea pig as a model for Brucella species infection. In the 1970s, Bosseray and Diaz inoculated pregnant guinea pigs via intramuscular (i.m.) inoculation with 5 × 104 B. abortus 544, which resulted in a 50% abortion rate (14). This study was exciting because it demonstrated that pregnant guinea pigs would develop reproductive disease when inoculated with a virulent Brucella spp.; however, the authors failed to specify the stage of gestation at the time of inoculation and did not culture the placenta. Additionally, i.m. inoculation is an artificial route of challenge and is less physiologically relevant for exploring the pathogenesis of reproductive disease. The current study confirms that abortions/stillbirths occur from Brucella melitensis secondary to aerosol inoculation, which is a means of transmission in naturally acquired brucellosis. Additionally, pregnant guinea pigs developed fever secondary to IT inoculation, further supporting the ability of this model to replicate natural disease. Therefore, the pregnant guinea pig model shows promise for evaluating the pathogenesis of Brucella-associated adverse pregnancy outcomes in women (14, 18).
While the pregnant guinea pig is a good model for brucellosis, research with guinea pigs does have limitations. With far fewer studies conducted in guinea pigs than in mice, limited reagents are commercially available to fully characterize the guinea pig immune response. However, the guinea pig genome has been sequenced and new annotations are continually added, indicating that a lack of reagents may become less of an impediment to using the guinea pig model. Another obstacle to completing these studies is the additional space required to house animals under BSL3 conditions. During pregnancy, guinea pigs weigh up to 1.5 kg and require larger cages and cage racks. These limitations may be why pregnant mice have been used more extensively to evaluate the tropism and effects of Brucella on the gravid uterus (11, 29–31). The mouse model also has the advantage of short generation time and a large number of commercially available reagents to evaluate the immunological response to infection (28).
An important goal of developing the pregnant guinea pig as a model for brucellosis is not only to have a better model for investigating the pathogenesis of reproductive disease, but also to have an animal model to evaluate vaccine candidates. A recent meta-analysis, which investigated the mouse model for Brucella vaccine development, found that the protection index has remained stable despite the development of novel subunit vaccines, DNA vaccines, and LAV mutants over the past 30 years (32). This suggests that while mice are an important animal model for investigating the pathogenesis of brucellosis, they may not be the best model for evaluating vaccines. As an example, Brucella ΔcydBA was attenuated in a BALB/c mouse model (33). When the same mutant was evaluated in pregnant goats, a natural host for B. melitensis, the LAV mutant colonized organs at the same level as the WT strain (34).
The guinea pig could prove a useful alternative animal model for developing and evaluating novel vaccines against brucellosis. Historically, guinea pigs were instrumental for developing and testing the safety and efficacy of commonly used Brucella vaccines, such as the B. melitensis mutant Rev. 1 and B. abortus S19 vaccines (35, 36). Guinea pigs were also used to compare the protection provided by the various vaccine strains against field isolates and were used to determine if vaccination against one strain offered cross protection (23, 37–40). Due to the similarities in disease manifestation, it was expected that vaccines or antigens which generate an active immune response in the guinea pig would be suitable for testing in humans and large animals (8, 35, 41, 42).
Previous studies have demonstrated that S19 is protective in a nonpregnant guinea pig challenge model, but efficacy in pregnant guinea pigs has not been evaluated (43). S19 was used because it is the reference strain against which new vaccines are compared, and it is classified as a BSL2 agent, allowing the breeding portion of the experiment to be conducted in group-housed open-bank caging available under BSL2 conditions (16, 44). Typically, Rev. 1 is used to protect small ruminants from infection with B. melitensis; however, Rev. 1 is classified as a select agent and vaccinated animals must be housed in a BSL3 level facility. This places a significant impediment on breeding experiments due to the strict caging regulations at BSL3 and associated increase in the animal numbers required. As the use of Rev. 1 in this context was not feasible, vaccine candidate B. melitensis 16MΔvjbR, which can be used under BSL2 conditions and has been extensively evaluated in a mouse model (12, 22), was used instead. Quil-A was added as an adjuvant because previous studies in mouse models using 16MΔvjbR suggested the need for additional immune stimulation to generate a robust response to vaccination (22). Quil-A promotes a cellular and humoral immune response and is on the list of approved adjuvants for use in human vaccines (45). The results of this study confirm the protective capacity of S19 and suggest that 16MΔvjbR requires additional modifications, such as adding a booster dose or a different adjuvant, to achieve a similar level of protection as S19. Even though guinea pigs were vaccinated with B. abortus LAV S19, vaccination was capable of providing protection against challenge with wild-type B. melitensis. Cross-protection between strains has previously been demonstrated in the guinea pig and mouse models, as well as in dairy cattle (23, 24, 38, 46).
Vaccination with both S19 and 16MΔvjbR generated a robust Brucella-specific IgG response. Interestingly, the IgG response to challenge in guinea pigs vaccinated with 16MΔvjbR + Quil-A was stronger than that of S19; however, this increase in IgG level did not correlate with superior protection against colonization or inflammation. While the humoral immune response is often used to evaluate the response to vaccination, the immune response to infection with Brucella spp. is largely dependent on the T cell response (47). In particular, several studies have demonstrated that vaccine protection relies on stimulating a strong Th1 response, with evidence that Th2 cells are less effective in controlling infection (48–50). The vaccine 16MΔvjbR induced a robust total IgG response but did not enhance protection, which suggests that vaccination with 16MΔvjbR may skew the humoral immune response toward a less effective IgG1/Th2 phenotype (50). However, we were unable to confirm this speculation due to a lack of a commercially available reagent for anti-guinea pig IgG1.
It is interesting to note that vaccination with S19 prevented fever following challenge with 16M, but the response of the guinea pig to vaccination with S19 was not evaluated. Previous reports of accidental exposure and a single trial using prisoner volunteers indicate that vaccination with BA-19 or Rev. 1 results in fever and symptoms of brucellosis in people (18, 44). As we did not monitor temperature during the period of vaccination, we are unable to conclude if vaccination with S19 or 16MΔvjbR produced fever in guinea pigs. Additional studies could illuminate this point and better define the guinea pig as a model for vaccine safety trials.
In conclusion, understanding the pathogenesis of brucellosis-associated reproductive disease is a crucial aspect for preventing disease in both humans and animals. Herein we present compelling data that the pregnant guinea pig is an excellent model for evaluating the events that underlie reproductive failure. The pregnant guinea pig also offers improvements upon the mouse model for vaccine studies because the two clinical endpoints of importance for human health, fever and adverse pregnancy events, are able to be evaluated in this model. Future studies using the pregnant guinea pig could be performed to develop novel therapeutics or vaccines. This study indicates that pregnant guinea pigs should be considered appropriate models to evaluate vaccine candidates in the pipeline because they can provide valuable information about clinical signs of infection, as well as the appropriate tissue targets to assess protection.
MATERIALS AND METHODS
Bacterial strains.
B. melitensis 16M (isolated from the lung of an aborted goat fetus), B. abortus S19 (National Veterinary Services Laboratory [NVSL], Ames, IA), and B. melitensis 16MΔvjbR (engineered for a previous study) were used in this study (22, 34). Bacteria were cultured on tryptic soy agar (TSA) (Difco, Becton Dickinson) at 37°C with 5% (vol/vol) CO2 for 72 h. B. melitensis 16M (WT) was harvested from plates with phosphate-buffered saline (PBS) (Gibco) and diluted to a final concentration of 1 × 107 CFU/50 μl using a Klett colorimeter and standard curve. S19 and 16MΔvjbR were similarly grown on TSA, harvested with PBS, and diluted to a final concentration of 1 × 109 CFU/100 μl. Final concentrations were retrospectively verified through serial dilution and plating onto TSA medium in duplicate.
Animal research ethics statement.
All studies were performed with the approval of the Texas A&M University’s Institutional Animal Care and Use Committee (protocol 2018-0046). Texas A&M University is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Kinetics of intratracheal inoculation.
Twenty-two multiparous pregnant Hartley guinea pigs at approximately 20 to 25 days of gestation (week 3 of gestation) were obtained from Charles River (Wilmington, MA) and housed individually in microisolator cages at an animal biosafety level 3 (ABSL-3) facility at Texas A&M University for the duration of the studies. After an acclimation period, animals were divided into four groups (n = 4) and were anesthetized intraperitoneally (i.p.) with a cocktail of ketamine (50 mg/kg) and xylazine (5 mg/kg). Implantable subcutaneous IPTT-300 microchips (BioMedic Data Systems, Seaford, DE) were placed to monitor body temperature. At 30 to 35 days of gestation, animals were then inoculated with 1 × 107 CFU/50 μl B. melitensis 16M using the PennCentury MicroSprayer aerosolizer (Wyndmoor, PA) as previously described (15). Briefly, a small animal laryngoscope was used to visualize the larynx and the blunt end of the MicroSprayer was inserted into the proximal trachea to deliver a dose of 50 μl.
Guinea pigs were evaluated daily for fever (body temperatures of ≥39.5°C) using a DAS-7000 reader (BioMedic Data Systems) (17) and were monitored twice daily for signs of reproductive failure, such as vaginal discharge or abortion.
At 7-day intervals representing gestation weeks 5 to 8, groups of pregnant guinea pigs (n = 4) were euthanized i.p. with sodium pentobarbital (100 mg/kg) followed by cardiac exsanguination. One gram each of spleen, liver, lung, left and right mammary gland, superficial inguinal lymph node, uterus, and placenta tissues was collected into presterilized 2-ml collection tubes containing 1 ml PBS and 1.47 g of ceramic beads (Omni International, Kennesaw, GA). Tissues were homogenized using a Bead Ruptor Elite Bead Mill Homogenizer (Omni International) for 30 s, serially diluted, and cultured on Farrell’s medium (TSA plus Oxoid Brucella selective supplement [Thermo Fisher Scientific, Waltham, MA], equine serum, and 20% dextrose) at 37°C with 5% (vol/vol) CO2 (15). After incubation for a minimum of 72 h, colonies were counted to determine CFU/g.
To evaluate the effect of infection on fetal development, fetuses were weighed and crown to rump (C-R) length was measured (19). Vertical transmission of infection from the dam to the offspring was evaluated by collecting liver, spleen, lung, and stomach contents from each fetus. Tissues were homogenized as described above, serially diluted, plated on Farrell’s medium, and incubated at 37°C with 5% (vol/vol) CO2 for 72 h. Colonies were counted to determine CFU/g.
Vaccination study.
Seventeen female Hartley guinea pigs at 6 to 7 weeks of age (Charles River) and six 450 to 500 g male Hartley guinea pigs (Charles River) were obtained and segregated by sex. Males were group housed at a BSL-1 animal facility prior to breeding. Female guinea pigs were individually housed in microisolator cages at a BSL-2 animal facility. Following an acclimation period of 5 days, female guinea pigs were randomly assigned to groups and were vaccinated by subcutaneous injection in the right inguinal region with 100 μl of 1 × 109 CFU/100 μl Brucella abortus S19 (n = 6) or Brucella melitensis 16MΔvjbR + 10 μg Quil-A (n = 6). Control animals were sham vaccinated with 100 μl sterile PBS + 10 μg Quil-A (n = 5).
Breeding.
Approximately 1 month after vaccination, female guinea pigs were segregated by vaccine group and cohoused with males in a BSL-2 level facility in open-bank cages for breeding purposes at a ratio of 3:1. After 2 weeks, females were evaluated daily by abdominal palpation to detect pregnancy (19). When guinea pigs were at approximately 30 to 35 days of gestation, they were moved to the BSL-3 facility, individually housed, and acclimated for a minimum of 2 days prior to challenge (Fig. S1).
Challenge of vaccinated pregnant guinea pigs with 1 × 107 CFU B. melitensis at 35 days of gestation.
Pregnant guinea pigs were anesthetized as described above, implanted with subcutaneous IPTT-300 microchips (BioMedic Data Systems), and challenged with 1 × 107 CFU B. melitensis 16M via IT inoculation. Euthanasia was performed 28 days postinoculation (p.i.) or at the time of parturition by sodium pentobarbital (100 mg/kg) followed by cardiac exsanguination. One gram each of placenta, uterus, liver, spleen, lung, superficial inguinal lymph node, and mammary gland tissues was collected for bacterial culture on Farrell’s medium as previously described. Fetal spleen, liver, lung, and stomach contents were collected for bacterial culture on Farrell’s medium.
Evaluation of histopathological changes.
From the adult guinea pigs, placenta, liver, spleen, lung, and left and right mammary gland samples were collected at the study endpoints and fixed in 10% neutral buffered formalin (NBF) (Thermo Scientific) for a minimum of 48 h. Fetal spleen, liver, lung, heart, kidney, reproductive tract, and umbilicus tissues were collected from each fetus and fixed in 10% NBF. Tissues were then routinely processed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). Histologic changes of the spleen, liver, placenta, and mammary gland were scored for inflammation type and severity in a blinded fashion by a board-certified veterinary pathologist (Table S1).
Immunohistochemistry to detect Brucella antigen.
Five-micrometer tissue sections of placenta and mammary gland were adhered to positively charged glass slides for immunohistochemistry. Slides were routinely processed through a series of xylene and ethanol steps before antigen retrieval was performed using 1:10 EMS Solution A (Electron Microscopy Services, Hatfield, PA) in a 2100 Antigen Retriever (Aptum Biologics Ltd., Southampton, UK), according to the manufacturer’s protocol. Endogenous peroxidase and alkaline phosphatase were blocked by 10 min of incubation with Bloxall blocking solution (Vector Laboratories, Burlingame, CA) followed by 20 min of blocking with normal goat serum (Vector Laboratories). Primary incubation was performed overnight at 4°C with Brucella polyclonal rabbit antibody (Bioss Antibodies, Woburn, MA) at a dilution of 1:800. As a negative control, tissue sections were incubated with rabbit nonimmune serum diluted in PBS. A Vectastain Elite ABC HRP kit (Vector Laboratories) with an avidin/biotinylated anti-rabbit secondary antibody was used according to the manufacturer’s instructions. Antigen was visualized with a Betazoid DAB chromogen kit (Biocare Medical, Pachecho, CA). The slides were counterstained with Meyer’s hematoxylin III.
Analysis of humoral immune response to infection by indirect ELISA.
Blood samples were collected from pregnant guinea pigs at days 0, 14, and 28 p.i. For the vaccination trial, serum samples were collected at 0, 4, 12, 14, and 16 weeks postvaccination. Indirect ELISA (iELISA) for anti-Brucella specific IgG was performed with guinea pig sera as previously described (15). Briefly, Nunc MaxiSorp 96-well plates (Thermo Fisher Scientific) were coated with 25 ng/well B. abortus 2308 heat-killed lysate and held overnight at 4°C. Plates were washed three times and blocked with 3% skim milk for 2 h at room temperature. Guinea pig sera were diluted to a concentration of 1:1,000, and 100 μl was added to plates and incubated at 37°C for 1 h. Plates were washed and goat anti-guinea pig IgG (H+L) (KPL, Seracare, Milford, MA) was added at a concentration of 1:2,000 and incubated at 37°C for 1 h. To detect peroxidase activity, SigmaFast OPD peroxidase substrate (Sigma-Aldrich, St. Louis, MO) was incubated for 30 min at 37°C, and absorbance was measured at 450 nm. Assays were performed in triplicate and results are presented as the mean value of three replicates.
Statistical analysis.
Statistical analysis was performed using two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons to evaluate the kinetics of infection. To determine vaccine efficacy, multiple comparisons between vaccinated and unvaccinated pregnant guinea pigs were performed using 2-way ANOVA followed by Tukey’s multiple comparisons. All tests were performed by using GraphPad Prism v6 (GraphPad Software, San Diego, CA).
Supplementary Material
ACKNOWLEDGMENTS
We thank K. Yeon Choi for sharing her expertise in guinea pig reproduction and pregnancy detection.
This study was supported by a National Institutes of Health Research Scientist Development Award (1K01TW009981) (to A.A.). Student stipend support (to M.E.H. and L.S.) was provided by a National Institutes of Health Institutional Training Grant T32 fellowship (5OD11083-7).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect Dis 6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 2.Pappas G, Panagopoulou P, Christou L, Akritidis N. 2006. Brucella as a biological weapon. Cell Mol Life Sci 63:2229–2236. doi: 10.1007/s00018-006-6311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbel MJ. 1997. Brucellosis: an overview. Emerg Infect Dis 3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan MY, Mah MW, Memish ZA. 2001. Brucellosis in pregnant women. Clin Infect Dis 32:1172–1177. doi: 10.1086/319758. [DOI] [PubMed] [Google Scholar]
- 5.Elshamy M, Ahmed AI. 2008. The effects of maternal brucellosis on pregnancy outcome. J Infect Dev Ctries 2:230–234. doi: 10.3855/jidc.268. [DOI] [PubMed] [Google Scholar]
- 6.Baud D, Greub G. 2011. Intracellular bacteria and adverse pregnancy outcomes. Clin Microbiol Infect 17:1312–1322. doi: 10.1111/j.1469-0691.2011.03604.x. [DOI] [PubMed] [Google Scholar]
- 7.Kurdoglu M, Adali E, Kurdoglu Z, Karahocagil MK, Kolusari A, Yildizhan R, Kucukaydin Z, Sahin HG, Kamaci M, Akdeniz H. 2010. Brucellosis in pregnancy: a 6-year clinical analysis. Arch Gynecol Obstet 281:201–206. doi: 10.1007/s00404-009-1106-0. [DOI] [PubMed] [Google Scholar]
- 8.García-Carrillo C. 1990. Laboratory animal models for brucellosis studies. CRC Press, Boca Raton, FL. [Google Scholar]
- 9.Silva TM, Costa EA, Paixao TA, Tsolis RM, Santos RL. 2011. Laboratory animal models for brucellosis research. J Biomed Biotechnol 2011:518323. doi: 10.1155/2011/518323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grillo MJ, Blasco JM, Gorvel JP, Moriyon I, Moreno E. 2012. What have we learned from brucellosis in the mouse model? Vet Res 43:29. doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Lee DS, Watanabe K, Furuoka H, Suzuki H, Watarai M. 2005. Interferon-gamma promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol 5:22. doi: 10.1186/1471-2180-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arenas-Gamboa AM, Rice-Ficht AC, Fan Y, Kahl-McDonagh MM, Ficht TA. 2012. Extended safety and efficacy studies of the attenuated Brucella vaccine candidates 16 M(Delta)vjbR and S19(Delta)vjbR in the immunocompromised IRF-1−/− mouse model. Clin Vaccine Immunol 19:249–260. doi: 10.1128/CVI.05321-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hensel ME, Arenas-Gamboa AM. 2018. A neglected animal model for a neglected disease: guinea pigs and the search for an improved animal model for human brucellosis. Front Microbiol 9:2593. doi: 10.3389/fmicb.2018.02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosseray N, Diaz R. 1974. Brucellose congenitale du cobaye. Ann de Recherches Veterinaires 5:147–153. [Google Scholar]
- 15.Hensel ME, Garcia-Gonzalez DG, Chaki SP, Samuel J, Arenas-Gamboa AM. 2019. Characterization of an intratracheal aerosol challenge model of Brucella melitensis in guinea pigs. PLoS One 14:e0212457. doi: 10.1371/journal.pone.0212457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garin-Bastuji B, Blasco JM. 2016. Brucellosis (infection with Brucella abortus, B. melitensis, and B. suis). http://www.oie.int/en/international-standard-setting/terrestrial-manual/access-online/.
- 17.Hawkins MG, Graham JE. 2007. Emergency and critical care of rodents. Vet Clin North Am Exot Anim Pract 10:501–531. doi: 10.1016/j.cvex.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Young EJ. 1983. Human brucellosis. Rev Infect Dis 5:821–842. doi: 10.1093/clinids/5.5.821. [DOI] [PubMed] [Google Scholar]
- 19.Kauffman PD, Davidoff M. 1977. The guinea-pig placenta. Advances in anatomy, embryology and cell biology, volume 53. Springer-Verlag, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 20.Mess A, Zaki N, Kadyrov M, Korr H, Kaufmann P. 2007. Caviomorph placentation as a model for trophoblast invasion. Placenta 28:1234–1238. doi: 10.1016/j.placenta.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Cossaboom CM, Kharod GA, Salzer JS, Tiller RV, Campbell LP, Wu K, Negron ME, Ayala N, Evert N, Radowicz J, Shuford J, Stonecipher S. 2018. Notes from the field: Brucella abortus vaccine strain RB51 infection and exposures associated with raw milk consumption—Wise County, Texas, 2017. MMWR Morb Mortal Wkly Rep 67:286. doi: 10.15585/mmwr.mm6709a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Rice-Ficht AC. 2008. Immunization with a single dose of a microencapsulated Brucella melitensis mutant enhances protection against wild-type challenge. Infect Immun 76:2448–2455. doi: 10.1128/IAI.00767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keppie J, Witt K, Smith H. 1963. Cross immunization of guinea-pigs with products of Brucellae abortus, melitensis and suis. Br J Exp Pathol 44:84–87. [PMC free article] [PubMed] [Google Scholar]
- 24.Jiménez de Baugés MP, Marín CM, Blasco JM. 1991. Effect of antibiotic therapy and strain-19 vaccination on the spread of Brucella melitensis within an infected dairy herd. Prev Vet Med 11:17–24. doi: 10.1016/S0167-5877(05)80041-8. [DOI] [Google Scholar]
- 25.Padilla-Carlin DJ, McMurray DN, Hickey AJ. 2008. The guinea pig as a model of infectious diseases. Comp Med 58:324–340. [PMC free article] [PubMed] [Google Scholar]
- 26.Byndloss MX, Tsai AY, Walker GT, Miller CN, Young BM, English BC, Seyffert N, Kerrinnes T, de Jong MF, Atluri VL, Winter MG, Celli J, Tsolis RM. 2019. Brucella abortus infection of placental trophoblasts triggers endoplasmic reticulum stress-mediated cell death and fetal loss via type IV secretion system-dependent activation of CHOP. mBio 10:e01538-19. doi: 10.1128/mBio.01538-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbier T, Machelart A, Zúñiga-Ripa A, Plovier H, Hougardy C, Lobet E, Willemart K, Muraille E, De Bolle X, Van Schaftingen E, Moriyón I, Letesson J-J. 2017. Erythritol availability in bovine, murine and human models highlights a potential role for the host aldose reductase during Brucella infection. Front Microbiol 8:1088. doi: 10.3389/fmicb.2017.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter AM. 2007. Animal models of human placentation—a review. Placenta 28:S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Bosseray N. 1980. Colonization of mouse placentas by Brucella abortus inoculated during pregnancy. Br J Exp Pathol 61:361–368. [PMC free article] [PubMed] [Google Scholar]
- 30.Bosseray N. 1983. Kinetics of placental colonization of mice inoculated intravenously with Brucella abortus at day 15 of pregnancy. Br J Exp Pathol 64:612–616. [PMC free article] [PubMed] [Google Scholar]
- 31.Tobias L, Cordes DO, Schurig GG. 1993. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Vet Pathol 30:119–129. doi: 10.1177/030098589303000204. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho TF, Haddad JP, Paixao TA, Santos RL. 2016. Meta-analysis and advancement of brucellosis vaccinology. PLoS One 11:e0166582. doi: 10.1371/journal.pone.0166582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endley S, McMurray D, Ficht TA. 2001. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J Bacteriol 183:2454–2462. doi: 10.1128/JB.183.8.2454-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahl-McDonagh MM, Elzer PH, Hagius SD, Walker JV, Perry QL, Seabury CM, den Hartigh AB, Tsolis RM, Adams LG, Davis DS, Ficht TA. 2006. Evaluation of novel Brucella melitensis unmarked deletion mutants for safety and efficacy in the goat model of brucellosis. Vaccine 24:5169–5177. doi: 10.1016/j.vaccine.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Herzberg M, Elberg SS. 1955. Immunization against Brucella infection. III. Response of mice and guinea pigs to injection of viable and nonviable suspensions of a streptomycin-dependent mutant of Brucella malitensis. J Bacteriol 69:432–435. doi: 10.1128/JB.69.4.432-435.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCamish J, Elberg SS. 1962. Immunization against Brucella infection: IX. The response of the guinea pig to the immunizing strain (Rev. 1) of Brucella melitensis. Am J Pathol 40:77–93. [PMC free article] [PubMed] [Google Scholar]
- 37.Jones LM, Thomson PD, Alton GG. 1958. Immunity against Brucella infection in guinea pigs produced by three different vaccines. J Comp Pathol 68:416–427. doi: 10.1016/s0368-1742(58)80047-8. [DOI] [PubMed] [Google Scholar]
- 38.Keppie J, Witt K, Smith H. 1972. The immunization of guinea-pigs and mice with a whole-culture extract of a smooth and a rough strain of Brucella abortus. Br J Exp Pathol 53:518–528. [PMC free article] [PubMed] [Google Scholar]
- 39.Woodard LF, Toone NM, Jasman RL. 1981. Brucella abortus vaccines: comparison of protection provided by immunopotentiated 45/20 bacterins and live strain 19 vaccine in guinea pigs. Am J Vet Res 42:1959–1962. [PubMed] [Google Scholar]
- 40.Hunter P, Pefanis SM, Williamson CC, Botha WJ, Van Schalkwyk MS. 1989. Horizontal transmission in sheep and delayed clearance in guinea pigs and mice of a Brucella melitensis Rev. I mutant. J S Afr Vet Assoc 60:92–94. [PubMed] [Google Scholar]
- 41.Huddleson IF. 1942. Immunity in brucellosis. Bacteriol Rev 6:111–142. doi: 10.1128/MMBR.6.2.111-142.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elberg S, Herzberg M, Schneider P, Silverman SJ, Meyer KF. 1951. Studies on the immunization of guinea pigs and mice to Brucella infection by means of the “native antigen”. J Immunol 67:1–13. [PubMed] [Google Scholar]
- 43.Khodzhaev S. 1959. A study of the postvaccination immunity of guinea pigs repeatedly inoculated with a strain of Brucella melitensis. Bull Exp Biol Med 47:486–490. doi: 10.1007/BF00779632. [DOI] [Google Scholar]
- 44.Spink WW, Hall JW 3rd, Finstad J, Mallet E. 1962. Immunization with viable Brucella organisms. Results of a safety test in humans. Bull World Health Organ 26:409–419. [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel FR. 2000. Improving vaccine performance with adjuvants. Clin Infect Dis 30 Suppl 3:S266–70. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 46.van Straten M, Bardenstein S, Keningswald G, Banai M. 2016. Brucella abortus S19 vaccine protects dairy cattle against natural infection with Brucella melitensis. Vaccine 34:5837–5839. doi: 10.1016/j.vaccine.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 47.de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. 2015. Pathogenesis and immunobiology of brucellosis: review of Brucella–host interactions. Am J Pathol 185:1505–1517. doi: 10.1016/j.ajpath.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eze MO, Yuan L, Crawford RM, Paranavitana CM, Hadfield TL, Bhattacharjee AK, Warren RL, Hoover DL. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect Immun 68:257–263. doi: 10.1128/iai.68.1.257-263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velikovsky CA, Goldbaum FA, Cassataro J, Estein S, Bowden RA, Bruno L, Fossati CA, Giambartolomei GH. 2003. Brucella lumazine synthase elicits a mixed Th1-Th2 immune response and reduces infection in mice challenged with Brucella abortus 544 independently of the adjuvant formulation used. Infect Immun 71:5750–5755. doi: 10.1128/iai.71.10.5750-5755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhan Y, Kelso A, Cheers C. 1995. Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect Immun 63:969–975. doi: 10.1128/IAI.63.3.969-975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.