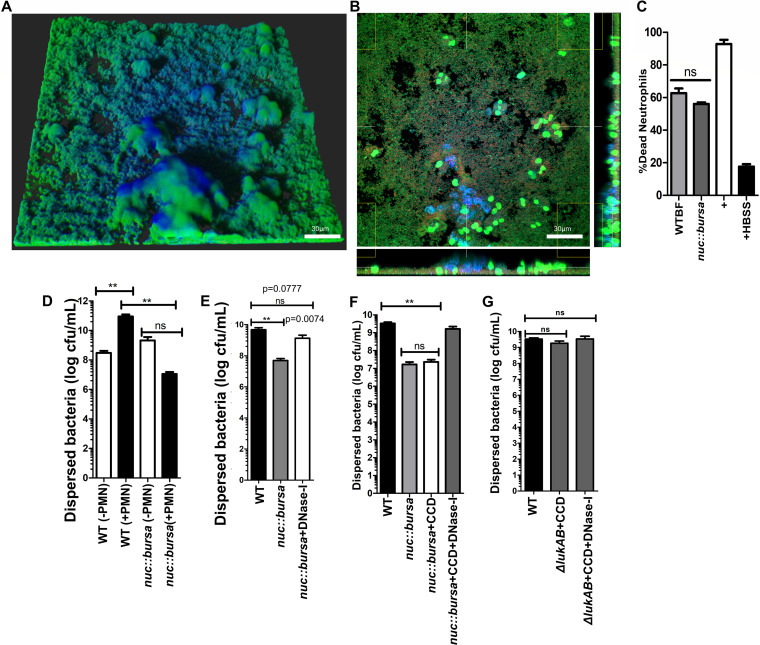
FIG 7.
Nuc-mediated breakdown of NET DNA contributes to biofilm bacterial dispersal. (A) nuc::bursa USA300 biofilms were treated with neutrophils for 1 h and subsequently with wild-type (WT) biofilm supernatant for an additional hour. Staining was done as described for Fig. 1. (B) Cross section of biofilm from panel A, incubated with neutrophils taken at the bottom of the biofilm. (C) Neutrophil killing activity of spent media collected from wild-type and nuc::bursa USA300 biofilms. Neutrophils were incubated with spent media for 30 min. LIVE-DEAD measurements show a ratio of Syto-9 (live) to propidium iodide fluorescence calculated as a percentage against a positive control (neutrophils plus 0.1% SDS). Neutrophils treated with Hanks balanced salt solution (HBSS) were used as a negative control. (D) Comparisons of dispersed bacteria before (−PMN) and after (+PMN) treatment of wild-type (WT) or nuc::bursa USA300 biofilms with neutrophils for 1 h. (E) Enumerations similar to those in panel D, comparing neutrophil-treated wild-type biofilms with nuc::bursa biofilms with and without DNase I treatment subsequent to incubation with neutrophils. (F) Counts (CFU per milliliter) of dispersed bacterial populations collected after a 60-min incubation of wild-type and nuc::bursa USA300 biofilms with primary human neutrophils, before and after treatment with cytochalasin D with and without DNase I. (G) Enumerations similar to those in panel F, performed with neutrophils treated with WT and ΔlukAB USA300 biofilms. Measurements of CFU per milliliter were done using biofilms grown in silicone tubing. Results are averages from six independent experiments performed in triplicate, with SEM. **, P < 0.01, using one-way analysis of variance and Tukey’s post hoc analysis; ns, not significant.