Abstract
Background:
Quality of life (QOL) is impaired in pancreatic cancer patients. Our aim was to investigate the determinants and prognostic value of QOL after diagnosis in a hospital-based cohort of racially/ethnically diverse patients with pancreatic ductal adenocarcinoma (PDAC).
Patients and methods:
QOL was prospectively assessed using the Short Form-12 in 2478 PDAC patients. The Physical Component Summary (PCS) and Mental Component Summary (MCS) were categorised into tertiles based on their distribution. Ordered logistic regression was adopted to compare the risk of having lower PCS and MCS by patient sociodemographic and clinical characteristics. The association of PCS and MCS with mortality was assessed by Cox regression.
Results:
Compared with non-Hispanic whites, Hispanics were at significantly higher risk of having lower PCS (odds ratio [95% CI], 1.69 [1.26–2.26]; P < 0.001) and lower MCS (1.66 [1.24–2.23]; P < 0.001). Patients diagnosed with stage III (1.80 [1.10–2.94]; P = 0.02) and stage IV (2.32 [1.50–3.59]; P < 0.001) PDAC were more likely to have lower PCS than stage I patients. Other determinants of QOL included sex, age, drinking, smoking, education level, comorbidities and time since diagnosis. The low tertile of PCS (hazard ratio [95% CI], 1.94 [1.72–2.18]; P < 0.001) and MCS (1.42 [1.26–1.59]; P < 0.001) were each related to poor prognosis. Similar results were found for non-Hispanic whites as compared with African-Americans/Hispanics/others.
Conclusion:
QOL after diagnosis is a significant prognostic indicator for patients with PDAC. Multiple factors determine QOL, suggesting possible means of intervention to improve QOL and outcomes of PDAC patients.
Keywords: Quality of life, Pancreatic ductal adenocarcinoma, Overall survival, Prognostic indicator, Short Form-12
1. Introduction
Pancreatic cancer (PC) is the third leading cause of cancer mortality in the United States [1] and the seventh globally [2]. In the United States, projections estimate that there will be 53,670 new cases of PC and 43,090 PC deaths in 2017 [1]. Pancreatic ductal adenocarcinoma (PDAC) accounts for 90% of all pancreatic cancers. The prognosis for patients with PDAC remains poor. The 5-year relative survival rate is 8% for all stages combined, 29% for local disease, and 3% for distant stage, respectively [3].
PDAC is known for its debilitating symptom burden and has a profound negative effect on patient quality of life (QOL) [4]. Consequently, QOL has become a subject of paramount importance for PDAC patients. Several studies of patients with PC have shown that higher baseline/pretreatment QOL is associated with longer overall survival [5–13], whereas another study showed no association [14]. However, these studies were limited by small sample sizes (ranging from 50 to 569), and most studies focused on metastatic or advanced-stage cancer without considering early-stage patients.
Identifying the determinants of QOL in PC patients could be important for clinicians to identify patients with poor QOL who need enhanced monitoring or improved care management. Previous studies have found some demographic (age) and clinical (clinical stage, operation type, and weight stabilisation) factors affect QOL in PC patients [15–17]. However, the sample sizes of these studies were also small and did not investigate the difference in determinants of QOL by race/ethnicity. Therefore, we assessed the prognostic value and the determinants of QOL after diagnosis in a large prospective cohort of racially/ethnically diverse patients with PDAC which encompassed all stages.
2. Methods
2.1. Patients
Participants were patients with histologically confirmed PDAC between August 1999 and October 2012 as part of The MD Anderson Cancer Patients and Survivors Cohort Study (MDA-CPSC) [18], a prospective hospital-based cohort study in the United States. At their initial visit, all participants completed a patient history form that collected epidemiologic, sociodemographic, and risk factor information. The patient history form also assessed QOL employing the generic, validated Short Form-12 vision 1 (SF-12v1) questionnaire [19]. Clinical information was abstracted from the institutional Tumour Registry. This study was approved by the institutional review board.
2.2. Eligibility and exclusion criteria
A total of 3725 PC patients completed the patient history form and SF-12v1 questionnaire within 1 year of diagnosis. We excluded patients who were younger than 18 years(N = 12), those who had been diagnosed with non-ductal adenocarcinoma (N = 789), those who had been diagnosed with multiple primary tumours (N = 442), and those who did not give the consents (N = 4). The final number of patients included in this study was 2478.
2.3. SF-12v1 questionnaire
The SF-12v1 questionnaire is a multipurpose generic QOL questionnaire evolved from the Short Form-36 questionnaire. The SF-12v1 questionnaire consists of 12 questions that measure 4 domains (physical, functional, emotional and social) and 8 subscales (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health). The 8 subscales of this tool can be summarised into 2 indices: the Physical Component Summary (PCS) and the Mental Component Summary (MCS), which describe the patient’s physical and mental well-being respectively [19]. Higher PCS and MCS scores indicated better QOL.
2.4. Statistical analysis
The PCS (high: ≥45.7, medium: 32.7–45.7, low: <32.7) and MCS (high: ≥52.3, medium: 40.3–52.3, low: <40.3) scores were categorised into tertiles based on the scores distribution. Ordered logistic regression was adopted to estimate the associations between patient characteristics and categorical PCS or MCS scores. First, each sociodemographic and clinical variable was independently assessed using a univariate model, with statistical significance set at P < 0.05. Next, variables found to be significant in the univariate analysis were included in a multivariate model, and forward selection was used to eliminate variables with a P value > 0.05. Because 1466 patients had missing stage data, we conducted a sensitivity analysis and found similar results when utilising the full data set and the reduced data set (only among those with stage information). Therefore, we presented the results from the full data set below.
Survival time was defined as the period from diagnosis to death or last follow-up. Cox proportional hazards models were adjusted for potential confounders (sex, age, marital status, race, education level, occupation, smoking, alcohol use, tumour size, cancer stage, comorbidity, treatment before survey, time since diagnosis and years of diagnosis). Survival estimates for the low, medium and high PCS and MCS groups were determined using the Kaplan–Meier method and compared using the log-rank test. All statistical tests were 2 sided, and P values < 0.05 were considered statistically significant. Statistical analyses were conducted using Stata 14.2 (StataCorp LP, College Station, Texas).
3. Results
3.1. Study population
The characteristics of the PDAC patients in this study are shown in Table 1. The study population, with a median age of 62.0 years (range: 28.0–90.0 years), consisted of 1489 (60.1%) males and 1966 (79.3%) non-Hispanic whites. Among the 1013 patients with stage information available, 533 (52.6%) were diagnosed with stage IV PDAC. Among the 577 (27.8%) patients who received treatment, 191 (33.1%) patients were treated by curative therapy (pancreatectomy with or without adjuvant treatment), 15 (2.6%) patients were treated by neoadjuvant therapy, 371 (64.3%) patients were treated by palliative treatment, and 56 (9.7%) patients were currently undergoing systemic therapy while surveyed. The mean of PCS and MCS was 38.9 (standard deviation: 11.6) and 45.3 (standard deviation: 10.7), respectively.
Table 1.
Association of patient characteristics with lower PCS score.
| Characteristic | N (%)a | PCS score |
Unadjusted |
P value | Adjustedb |
P value | ||
|---|---|---|---|---|---|---|---|---|
| ≥45.7 | 32.7–45.7 | <32.7 | OR (95% CI) | OR (95% CI) | ||||
| Sex | ||||||||
| Male | 1,489 (60.1) | 498 | 517 | 474 | 1.00 (Ref) | 1.00 (Ref) | ||
| Female | 989 (39.9) | 294 | 325 | 370 | 1.23 (1.06–1.43) | 0.005 | 1.10 (0.93–1.30) | 0.29 |
| Age at diagnosis, yrs | ||||||||
| 18–44 | 162 (6.5) | 45 | 56 | 61 | 1.00 (Ref) | 1.00 (Ref) | ||
| 45–54 | 534 (21.5) | 165 | 197 | 172 | 0.82 (0.60–1.14) | 0.24 | 0.83 (0.59–1.17) | 0.29 |
| 55–64 | 888 (35.8) | 306 | 303 | 279 | 0.75 (0.55–1.02) | 0.06 | 0.76 (0.54–1.05) | 0.10 |
| 65–74 | 651 (26.3) | 207 | 214 | 230 | 0.87 (0.63–1.19) | 0.38 | 0.91 (0.65–1.29) | 0.60 |
| ≥75 | 243 (9.8) | 69 | 72 | 102 | 1.10 (0.76–1.59) | 0.61 | 1.06 (0.71–1.60) | 0.77 |
| P for trend | 0.29 | 0.35 | ||||||
| Marital status | ||||||||
| Married | 1952 (78.8) | 640 | 684 | 628 | 1.00 (Ref) | 1.00 (Ref) | ||
| Never married | 203 (8.2) | 61 | 66 | 76 | 1.20 (0.92–1.57) | 0.18 | 1.10 (0.83–1.46) | 0.50 |
| Divorced | 138 (5.6) | 44 | 38 | 56 | 1.25 (0.90–1.73) | 0.18 | 1.11 (0.79–1.56) | 0.55 |
| Widowed | 167 (6.7) | 42 | 51 | 74 | 1.58 (1.18–2.13) | 0.002 | 1.10 (0.79–1.54) | 0.58 |
| Others | 18 (0.7) | 5 | 3 | 10 | 2.09 (0.83–5.29) | 0.12 | 2.91 (1.13–7.51) | 0.03 |
| Race | ||||||||
| Non-Hispanic whites | 1966 (79.3) | 678 | 655 | 633 | 1.00 (Ref) | 1.00 (Ref) | ||
| African-Americans | 166 (6.7) | 34 | 54 | 78 | 1.94 (1.44–2.61) | <0.001 | 1.33 (0.97–1.83) | 0.07 |
| Hispanics | 186 (7.5) | 34 | 72 | 80 | 1.81 (1.38–2.39) | <0.001 | 1.69 (1.26–2.26) | <0.001 |
| Others | 160 (6.5) | 46 | 61 | 53 | 1.16 (0.87–1.55) | 0.32 | 1.01 (0.74–1.39) | 0.93 |
| Education | ||||||||
| < High school | 171 (6.9) | 29 | 55 | 87 | 1.00 (Ref) | 1.00 (Ref) | ||
| High school/vocational | 681 (27.5) | 190 | 199 | 292 | 0.65 (0.47–0.89) | 0.008 | 0.80 (0.57–1.12) | 0.19 |
| ≥ College degree | 1508 (60.9) | 540 | 545 | 423 | 0.38 (0.28–0.52) | <0.001 | 0.59 (0.42–0.83) | <0.001 |
| Unknown | 118 (4.8) | 33 | 43 | 42 | 0.54 (0.35–0.84) | 0.006 | 0.69 (0.43–1.10) | 0.12 |
| P for trend | <0.001 | <0.001 | ||||||
| Occupation | ||||||||
| White collar | 914 (36.9) | 307 | 339 | 268 | 1.00 (Ref) | 1(reference) | ||
| Blue collar | 311 (12.6) | 81 | 114 | 116 | 1.41 (1.12–1.78) | 0.004 | 1.12 (0.86–1.45) | 0.40 |
| Others | 164 (6.6) | 55 | 56 | 53 | 1.07 (0.79–1.45) | 0.67 | 0.83 (0.60–1.15) | 0.26 |
| Unknown | 1089 (43.9) | 349 | 333 | 407 | 1.24 (1.06–1.46) | 0.008 | 1.10 (0.92–1.31) | 0.30 |
| Smoking status | ||||||||
| Never | 1122 (45.6) | 373 | 382 | 367 | 1.00 (Ref) | 1.00 (Ref) | ||
| Former | 1056 (42.9) | 345 | 367 | 344 | 1.01 (0.87–1.18) | 0.90 | 1.07 (0.91–1.26) | 0.43 |
| Current | 282 (11.5) | 72 | 83 | 127 | 1.60 (1.25–2.04) | <0.001 | 1.59 (1.23–2.06) | <0.001 |
| P for trend | 0.004 | 0.003 | ||||||
| Alcohol use | ||||||||
| Never | 1030 (41.8) | 275 | 329 | 426 | 1.00 (Ref) | 1.00 (Ref) | ||
| Former | 671 (27.3) | 171 | 248 | 252 | 0.93 (0.78–1.11) | 0.44 | 0.93 (0.77–1.13) | 0.47 |
| Current | 761 (30.9) | 344 | 255 | 162 | 0.41 (0.34–0.49) | <0.001 | 0.46 (0.38–0.55) | <0.001 |
| P for trend | <0.001 | <0.001 | ||||||
| Tumour size | ||||||||
| 0–20 mm | 105 (4.2) | 44 | 35 | 26 | 1.00 (Ref) | 1.00 (Ref) | ||
| 21–30 mm | 233 (9.4) | 94 | 81 | 58 | 1.04 (0.68–1.60) | 0.85 | 1.20 (0.77–1.88) | 0.42 |
| >30 mm | 526 (21.2) | 173 | 174 | 179 | 1.52 (1.03–2.24) | 0.04 | 1.40 (0.92–2.13) | 0.11 |
| Unknown | 1614 (65.1) | 481 | 552 | 581 | 1.70 (1.18–2.45) | 0.004 | 1.49 (0.99–2.25) | 0.06 |
| P for trend | <0.001 | 0.04 | ||||||
| AJCC cancer stage | ||||||||
| I | 97 (3.9) | 45 | 33 | 19 | 1.00 (Ref) | 1.00 (Ref) | ||
| II | 221 (8.9) | 103 | 66 | 52 | 1.07 (0.69–1.67) | 0.77 | 1.08 (0.68–1.72) | 0.75 |
| III | 162 (6.5) | 58 | 54 | 50 | 1.64 (1.03–2.62) | 0.04 | 1.80 (1.10–2.94) | 0.02 |
| IV | 533 (21.5) | 140 | 184 | 209 | 2.47 (1.65–3.69) | <0.001 | 2.32 (1.50–3.59) | <0.001 |
| Unknown | 1465 (59.1) | 446 | 505 | 514 | 2.05 (1.40–3.00) | <0.001 | 1.72 (1.12–2.65) | 0.01 |
| P for trend | <0.001 | <0.001 | ||||||
| Metastatic site(s) | ||||||||
| 1 | 239 (44.8) | 64 | 76 | 99 | 1.00 (Ref) | |||
| ≥2 | 294 (55.2) | 76 | 108 | 110 | 0.92 (0.67–1.26) | 0.61 | 0.89 (0.64–1.25) | 0.52 |
| Comorbidity | ||||||||
| No | 721 (29.1) | 268 | 255 | 198 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 1757 (70.9) | 524 | 587 | 646 | 1.46 (1.24–1.71) | <0.001 | 1.39 (1.17–1.65) | <0.001 |
| Heart disease | ||||||||
| No | 2003 (80.8) | 666 | 679 | 658 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 475 (19.2) | 126 | 163 | 186 | 1.30 (1.06–1.60) | 0.002 | 1.30 (1.06–1.60) | 0.01 |
| Lung disease | ||||||||
| No | 2281 (92.1) | 751 | 783 | 747 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 197 (7.9) | 41 | 59 | 97 | 1.95 (1.48–2.57) | <0.001 | 1.86 (1.39–2.48) | <0.001 |
| Diabetes | ||||||||
| No | 1825 (73.6) | 635 | 625 | 565 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 653 (26.4) | 157 | 217 | 279 | 1.67 (1.42–1.97) | <0.001 | 1.43 (1.19–1.72) | <0.001 |
| Hypertension | ||||||||
| No | 1343 (54.2) | 470 | 449 | 424 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 1135 (45.8) | 322 | 393 | 420 | 1.31 (1.14–1.52) | <0.001 | 1.11 (0.92–1.34) | 0.27 |
| Liver disease | ||||||||
| No | 2343 (94.6) | 759 | 796 | 788 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 135 (5.4) | 33 | 46 | 56 | 1.43 (1.04–1.97) | 0.03 | 1.06 (0.76–1.49) | 0.72 |
| Renal disease | ||||||||
| No | 2210 (89.0) | 729 | 756 | 725 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 268 (10.8) | 63 | 86 | 119 | 1.62 (1.28–2.06) | <0.001 | 1.48 (1.14–1.91) | <0.001 |
| Infectious disease | ||||||||
| No | 2439 (98.4) | 786 | 829 | 824 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 39 (1.6) | 6 | 13 | 20 | 2.19 (1.21–3.97) | 0.01 | 1.85 (0.99–3.43) | 0.05 |
| Stroke | ||||||||
| No | 2397 (96.7) | 786 | 812 | 799 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 81 (3.3) | 6 | 30 | 45 | 2.89 (1.90–4.39) | <0.001 | 2.57 (1.65–3.99) | <0.001 |
| Digestive tract bleeding | ||||||||
| No | 2420 (97.7) | 780 | 825 | 815 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 58 (2.3) | 12 | 17 | 29 | 1.93 (1.17–3.16) | 0.01 | 1.55 (0.93–2.60) | 0.09 |
| Seizure | ||||||||
| No | 2449 (98.8) | 786 | 835 | 828 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 29 (1.2) | 6 | 7 | 16 | 2.24 (1.09–4.57) | 0.03 | 1.83 (0.87–3.83) | 0.11 |
| Time since diagnosisc | ||||||||
| < 1 month | 1174 (47.4) | 429 | 375 | 370 | 1.00 (Ref) | 1.00 (Ref) | ||
| 1–3 months | 890 (35.9) | 226 | 326 | 338 | 1.49 (1.27–1.74) | <0.001 | 1.27 (1.07–1.52) | 0.007 |
| 3–6 months | 218 (8.8) | 64 | 72 | 82 | 1.36 (1.04–1.77) | 0.03 | 1.10 (0.79–1.52) | 0.58 |
| ≥ 6 months | 196 (7.9) | 73 | 69 | 54 | 0.90 (0.68–1.19) | 0.47 | 0.83 (0.59–1.16) | 0.28 |
| P for trend | 0.17 | 0.99 | ||||||
| Years of diagnosis | ||||||||
| 1999–2001 | 218 (8.8) | 59 | 79 | 80 | 1.00 (Ref) | 1.00 (Ref) | ||
| 2002–2004 | 355 (14.3) | 109 | 110 | 136 | 0.96 (0.71–1.31) | 0.81 | 0.97 (0.70–1.34) | 0.86 |
| 2005–2007 | 632 (25.5) | 204 | 215 | 213 | 0.83 (0.63–1.10) | 0.20 | 0.87 (0.64–1.17) | 0.35 |
| 2008–2010 | 820 (33.1) | 269 | 275 | 276 | 0.82 (0.63–1.08) | 0.16 | 0.89 (0.66–1.18) | 0.41 |
| 2011–2012 | 453 (18.3) | 151 | 163 | 139 | 0.76 (0.57–1.02) | 0.07 | 0.87 (0.64–1.20) | 0.40 |
| P for trend | 0.03 | 0.34 | ||||||
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; OR, odds ratio; PCS, Physical Component Summary.
Missing values not included: smoking status (N = 18); alcohol status (N = 16), percentages may not add up to 100% due to rounding.
Adjusted for sex, age, marital status, race, education level, occupation, smoking, alcohol use, tumour size, cancer stage, comorbidity, treatment before survey, time since diagnosis and years of diagnosis if appropriate.
The interval between initial diagnosis and quality-of-life survey.
3.2. Risk factors for lower PCS and MCS
We assessed the association between patient characteristics and PCS (Table 1) or MCS (Table 2) scores which were categorised into tertiles. In multivariate analysis, Hispanic ethnicity, low education level, presence of comorbidity were all significantly associated with poorer PCS and MCS. Specially, individuals reporting Hispanic ethnicity had a 1.69-fold (odds ratio [OR] 95% confidence interval [95% CI], 1.69 [1.26–2.26]; P < 0.001) increased risk of lower PCS and a 1.66-fold (1.66 [1.24–2.23]; P < 0.001) increased risk of lower MCS than did non-Hispanic whites. Patients with college degree or above were more likely to have higher PCS (0.59 [0.42–0.83]; P < 0.001) and MCS (0.71 [0.51–0.98]; P = 0.04) than were patients with less than high school attainment. Patients with comorbidities were more likely to have lower PCS (1.39 [1.17–1.65]; P < 0.001) and MCS (1.22 [1.03–1.44]; P = 0.02) than were patients with no comorbidities.
Table 2.
Association of patient characteristics with lower MCS score.
| Characteristic | N (%)a | MCS score |
Unadjusted |
P value | Adjustedb |
P value | ||
|---|---|---|---|---|---|---|---|---|
| ≥52.3 | 40.3–52.3 | <40.3 | OR (95% CI) | OR (95% CI) | ||||
| Sex | ||||||||
| Male | 1489 (60.1) | 517 | 512 | 460 | 1.00 (Ref) | 1.00 (Ref) | ||
| Female | 989 (39.9) | 278 | 334 | 377 | 1.37 (1.18–1.59) | <0.001 | 1.37 (1.16–1.64) | <0.001 |
| Age at diagnosis, yrs | ||||||||
| 18–44 | 162 (6.5) | 42 | 59 | 61 | 1.00 (Ref) | 1.00 (Ref) | ||
| 45–54 | 534 (21.5) | 168 | 179 | 187 | 0.83 (0.61–1.15) | 0.27 | 0.81 (0.58–1.13) | 0.21 |
| 55–64 | 888 (35.8) | 257 | 332 | 299 | 0.85 (0.63–1.16) | 0.31 | 0.82 (0.59–1.13) | 0.23 |
| 65–74 | 651 (26.3) | 232 | 208 | 211 | 0.71 (0.52–0.98) | 0.04 | 0.66 (0.47–0.93) | 0.02 |
| ≥75 | 243 (9.8) | 96 | 68 | 79 | 0.65 (0.45–0.94) | 0.02 | 0.56 (0.37–0.84) | 0.005 |
| P for trend | 0.006 | 0.001 | ||||||
| Marital status | ||||||||
| Married | 1952 (78.8) | 637 | 691 | 624 | 1.00 (Ref) | 1.00 (Ref) | ||
| Never married | 203 (8.2) | 62 | 65 | 76 | 1.19 (0.91–1.56) | 0.20 | 1.03 (0.78–1.36) | 0.82 |
| Divorced | 138 (5.6) | 40 | 36 | 62 | 1.49 (1.07–2.07) | 0.02 | 1.37 (0.97–1.92) | 0.07 |
| Widowed | 167 (6.7) | 52 | 48 | 67 | 1.26 (0.93–1.69) | 0.13 | 1.11 (0.79–1.54) | 0.55 |
| Others | 18 (0.7) | 4 | 6 | 8 | 1.69 (0.71–3.99) | 0.24 | 1.81 (0.75–4.39) | 0.19 |
| Race | ||||||||
| Non-Hispanic whites | 1966 (79.3) | 656 | 670 | 640 | 1.00 (Ref) | 1.00 (Ref) | ||
| African-Americans | 166 (6.7) | 43 | 52 | 71 | 1.51 (1.12–2.02) | 0.007 | 1.24 (0.91–1.68) | 0.18 |
| Hispanics | 186 (7.5) | 40 | 62 | 84 | 1.75 (1.32–2.31) | <0.001 | 1.66 (1.24–2.23) | <0.001 |
| Others | 160 (6.5) | 56 | 62 | 42 | 0.84 (0.63–1.13) | 0.24 | 0.82 (0.60–1.12) | 0.22 |
| Education | ||||||||
| < High school | 171 (6.9) | 43 | 47 | 81 | 1.00 (Ref) | 1.00 (Ref) | ||
| High school/vocational | 681 (27.5) | 205 | 218 | 258 | 0.70 (0.51–0.97) | 0.03 | 0.81 (0.58–1.13) | 0.22 |
| ≥ College degree | 1508 (60.9) | 509 | 545 | 454 | 0.54 (0.40–0.73) | <0.001 | 0.71 (0.51–0.98) | 0.04 |
| Unknown | 118 (4.8) | 38 | 36 | 44 | 0.66 (0.43–1.03) | 0.07 | 0.79 (0.50–1.26) | 0.32 |
| P for trend | <0.001 | 0.06 | ||||||
| Occupation | ||||||||
| White collar | 914 (36.9) | 302 | 321 | 291 | 1.00 (Ref) | 1.00 (Ref) | ||
| Blue collar | 311 (12.6) | 95 | 103 | 113 | 1.17 (0.93–1.49) | 0.19 | 1.07 (0.83–1.39) | 0.58 |
| Others | 164 (6.6) | 56 | 52 | 56 | 1.03 (0.76–1.40) | 0.86 | 0.89 (0.64–1.24) | 0.50 |
| Unknown | 1089 (43.9) | 342 | 370 | 377 | 1.11 (0.94–1.30) | 0.22 | 1.07 (0.90–1.27) | 0.47 |
| Smoking status | ||||||||
| Never | 1122 (45.6) | 368 | 398 | 356 | 1.00 (Ref) | 1.00 (Ref) | ||
| Former | 1056 (42.9) | 331 | 372 | 353 | 1.07 (0.92–1.25) | 0.37 | 1.09 (0.92–1.28) | 0.33 |
| Current | 282 (11.5) | 90 | 70 | 122 | 1.35 (1.05–1.73) | 0.02 | 1.15 (0.89–1.49) | 0.29 |
| P for trend | 0.03 | 0.21 | ||||||
| Alcohol use | ||||||||
| Never | 1030 (41.8) | 326 | 357 | 347 | 1.00 (Ref) | 1.00 (Ref) | ||
| Former | 671 (27.3) | 177 | 216 | 278 | 1.35 (1.13–1.62) | 0.001 | 1.44 (1.18–1.75) | <0.001 |
| Current | 761 (30.9) | 288 | 265 | 208 | 0.75 (0.63–0.89) | 0.001 | 0.84 (0.70–1.01) | 0.07 |
| P for trend | 0.005 | 0.08 | ||||||
| Tumour size | ||||||||
| 0–20 mm | 105 (4.2) | 34 | 34 | 37 | 1.00 (Ref) | 1.00 (Ref) | ||
| 21–30 mm | 233 (9.4) | 82 | 80 | 71 | 0.84 (0.55–1.29) | 0.43 | 0.92 (0.60–1.43) | 0.71 |
| >30 mm | 526 (21.2) | 165 | 182 | 179 | 0.99 (0.68–1.46) | 0.98 | 1.02 (0.68–1.54) | 0.92 |
| Unknown | 1614 (65.1) | 514 | 550 | 550 | 0.99 (0.68–1.42) | 0.94 | 1.06 (0.71–1.59) | 0.77 |
| P for trend | 0.53 | 0.45 | ||||||
| AJCC cancer stage | ||||||||
| I | 97 (3.9) | 36 | 29 | 32 | 1.00 (Ref) | 1.00 (Ref) | ||
| II | 221 (8.9) | 95 | 59 | 67 | 0.81 (0.52–1.27) | 0.36 | 0.80 (0.50–1.27) | 0.34 |
| III | 162 (6.5) | 45 | 52 | 65 | 1.46 (0.92–2.34) | 0.11 | 1.57 (0.96–2.55) | 0.07 |
| IV | 533 (21.5) | 159 | 187 | 187 | 1.24 (0.83–1.86) | 0.30 | 1.16 (0.75–1.78) | 0.51 |
| Unknown | 1465 (59.1) | 460 | 519 | 486 | 1.15 (0.78–1.68) | 0.49 | 1.22 (0.80–1.87) | 0.36 |
| P for trend | 0.16 | 0.07 | ||||||
| Metastatic site(s) | ||||||||
| 1 | 239 (44.8) | 70 | 83 | 86 | 1.00 (Ref) | 1.00 (Ref) | ||
| ≥2 | 294 (55.2) | 89 | 104 | 101 | 0.94 (0.69–1.29) | 0.71 | 0.98 (0.70–1.36) | 0.90 |
| Comorbidity | ||||||||
| No | 721 (29.1) | 240 | 262 | 219 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 1757 (70.9) | 555 | 584 | 618 | 1.16 (0.99–1.36) | 0.07 | 1.22 (1.03–1.44) | 0.02 |
| Heart disease | ||||||||
| No | 2003 (80.8) | 646 | 706 | 651 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 475 (19.2) | 149 | 140 | 186 | 1.19 (0.99–1.44) | 0.06 | 1.37 (1.11–1.68) | 0.003 |
| Lung disease | ||||||||
| No | 2281 (92.1) | 737 | 782 | 762 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 197 (7.9) | 58 | 64 | 75 | 1.19 (0.91–1.56) | 0.21 | 1.15 (0.87–1.53) | 0.31 |
| Diabetes | ||||||||
| No | 1825 (73.6) | 584 | 630 | 611 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 653 (26.4) | 211 | 216 | 226 | 1.02 (0.86–1.20) | 0.82 | 0.92 (0.77–1.11) | 0.40 |
| Hypertension | ||||||||
| No | 1343 (54.2) | 432 | 477 | 434 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 1135 (45.8) | 363 | 369 | 403 | 1.08 (0.93–1.25) | 0.30 | 1.01 (0.84–1.21) | 0.95 |
| Liver disease | ||||||||
| No | 2343 (94.6) | 762 | 802 | 779 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 135 (5.4) | 33 | 44 | 58 | 1.50 (1.09–2.08) | 0.01 | 1.32 (0.94–1.84) | 0.11 |
| Renal disease | ||||||||
| No | 2210 (89.0) | 710 | 755 | 745 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 268 (10.8) | 85 | 91 | 92 | 1.02 (0.81–1.29) | 0.84 | 1.04 (0.81–1.33) | 0.78 |
| Infectious disease | ||||||||
| No | 2439 (98.4) | 787 | 835 | 817 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 39 (1.6) | 8 | 11 | 20 | 2.02 (1.10–3.69) | 0.02 | 1.75 (0.94–3.25) | 0.08 |
| Stroke | ||||||||
| No | 2397 (96.7) | 775 | 817 | 805 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 81 (3.3) | 20 | 29 | 32 | 1.35 (0.90–2.03) | 0.14 | 1.30 (0.85–1.98) | 0.22 |
| Digestive tract bleeding | ||||||||
| No | 2420 (97.7) | 779 | 825 | 816 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 58 (2.3) | 16 | 21 | 21 | 1.17 (0.73–1.88) | 0.52 | 1.07 (0.66–1.76) | 0.77 |
| Seizure | ||||||||
| No | 2449 (98.8) | 787 | 835 | 827 | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 29 (1.2) | 8 | 11 | 10 | 1.12 (0.58–2.18) | 0.73 | 0.98 (0.50–1.93) | 0.96 |
| Time since diagnosisc | ||||||||
| < 1 month | 1174 (47.4) | 356 | 432 | 386 | 1.00 (Ref) | 1.00 (Ref) | ||
| 1–3 months | 890 (35.9) | 289 | 276 | 325 | 1.04 (0.88–1.22) | 0.65 | 0.99 (0.83–1.18) | 0.92 |
| 3–6 months | 218 (8.8) | 83 | 66 | 69 | 0.81 (0.62–1.06) | 0.13 | 0.92 (0.66–1.28) | 0.61 |
| ≥ 6 months | 196 (7.9) | 67 | 72 | 57 | 0.84 (0.64–1.11) | 0.23 | 1.05 (0.75–1.46) | 0.77 |
| P for trend | 0.14 | 0.94 | ||||||
| Years of diagnosis | ||||||||
| 1999–2001 | 218 (8.8) | 57 | 73 | 88 | 1.00 (Ref) | 1.00 (Ref) | ||
| 2002–2004 | 355 (14.3) | 111 | 132 | 112 | 0.73 (0.53–0.99) | 0.04 | 0.72 (0.52–0.99) | 0.05 |
| 2005–2007 | 632 (25.5) | 204 | 195 | 233 | 0.80 (0.61–1.07) | 0.14 | 0.88 (0.65–1.19) | 0.41 |
| 2008–2010 | 820 (33.1) | 267 | 288 | 265 | 0.72 (0.55–0.95) | 0.02 | 0.78 (0.59–1.05) | 0.10 |
| 2011–2012 | 453 (18.3) | 156 | 158 | 139 | 0.66 (0.49–0.90) | 0.007 | 0.75 (0.55–1.03) | 0.08 |
| P for trend | 0.02 | 0.24 | ||||||
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; MCS, Mental Component Summary; OR, odds ratio.
Missing values not included: smoking status (N = 18); alcohol status (N = 16), percentages may not add up to 100% due to rounding.
Adjusted for sex, age, marital status, race, education level, occupation, smoking, alcohol use, tumour size, cancer stage, comorbidity, treatment before survey, time since diagnosis and years of diagnosis if appropriate.
The interval between initial diagnosis and quality-of-life survey.
Smoking, alcohol use, tumour stage and time since diagnosis were significantly associated with PCS. Specially, current smokers carried a 1.59-fold (1.59 [1.23–2.06]; P < 0.001) increased risk of lower PCS than did never-smokers. Current alcohol drinkers were more likely to have higher PCS (0.46 [0.38–0.55]; P < 0.001) than were patients who never consumed alcohol. Patients diagnosed with stage III (1.80 [1.10–2.94]; P = 0.02) and stage IV (2.32 [1.50–3.59]; P < 0.001) were more likely to have lower PCS than were patients diagnosed with stage I (P for trend < 0.001). Compared to patients diagnosed within one month, those diagnosed from one to three months carried an increased risk of low PCS (1.27 [1.07–1.52]; P = 0.007).
Sex and age at diagnosis were significantly associated with MCS. Specially, female patients had a significantly elevated risk of lower MCS than did male patients (1.37 [1.16–1.64]; P < 0.001). Patients aged from 65 to 74 years (0.66 [0.47–0.93]; P = 0.02) and 75 years and over (0.56 [0.37–0.84]; P = 0.005) carried reduced risk of lower MCS. Our study also showed a trend for improved PCS and MCS by years of diagnosis (all ORs < 1.0, P for trend pcs = 0.03; P for trend mcs = 0.02) in univariate analysis. However, the association was not statistically significant in multivariate analysis. Similar results were found across different race/ethnicity strata (Supplemental Tables 1 and 2).
3.3. Association of PCS and MCS with survival
The median follow-up time was 60.2 months (95% CI: 52.5–64.1 months). The median survival time for all patients was 12.5 months (95% CI: 12.0–13.0 months). The overall 1-year and 5-year relative survival rates for all patients were 52.1% and 8.1%, respectively.
Differences in the overall survival by PCS or MCS scores are shown in Table 3, Figs. 1 and 2. We found that patients with low-PCS and medium-PCS had a significantly reduced survival rate than did patients in the high-PCS group (log-rank P < 0.001; Fig. 1A). After adjustment for sex, age, marital status, race, education level, occupation, smoking, alcohol use, tumour size, cancer stage, comorbidity, treatment before survey, time since diagnosis and years of diagnosis, patients in the low-PCS (hazard ratio [95% CI], 1.94 [1.72–2.18]; P < 0.001) and medium-PCS (1.37 [1.22–1.53]; P < 0.001) groups had significantly increased risk of death than did patients in the high-PCS group. Similarly, patients in the low-MCS and medium-MCS groups had significantly reduced survival rate (log-rank P < 0.001; Fig. 2A) and carried a 1.42-fold (1.42 [1.26–1.59]; P < 0.001) and a 1.26-fold (1.26 [1.12–1.41]; P < 0.001) increased risk of dying than did patients in the high-MCS group. To assess any possible bias stemming from the effects of missing disease stage, we repeated the analysis for the 1013 patients with stage information available, and we observed similar results (Figs. 1B and 2B). When further stratified by stage, this effect of PCS on overall survival was consistent between early- and late-stage patients (Fig. 1C and D). However, no significant association of MCS with survival was found in stage I, II PDAC (Fig. 2C). We also repeated the analysis stratified by race/ethnicity and treatment before survey history, the impact of lower PCS and MCS on survival was consistent for non-Hispanic whites as compared with African-Americans/Hispanics/others and patients without treatment before survey comparing to those with treatment before survey (Supplemental Figures 1, 2, 3, and 4).
Table 3.
Association of PCS/MCS score with five-year survival.
| SF-12 score | Dead (N) | Alive (N) | HR (95% CI)a | P value | MST (month) | Log rank P |
|---|---|---|---|---|---|---|
| All patients | ||||||
| PCS | ||||||
| ≥45.7 | 562 | 230 | 1.00 (Ref) | 16.6 | ||
| 32.7–45.7 | 685 | 157 | 1.37 (1.22–1.53) | <0.001 | 12.7 | |
| <32.7 | 694 | 150 | 1.94 (1.72–2.18) | <0.001 | 9.2 | <0.001 |
| P for trend | 1.39 (1.31–1.48) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 594 | 201 | 1.00 (Ref) | 14.8 | ||
| 40.3–52.3 | 664 | 182 | 1.26 (1.12–1.41) | <0.001 | 12.4 | |
| <40.3 | 683 | 154 | 1.42 (1.26–1.59) | <0.001 | 10.4 | <0.001 |
| P for trend | 1.19 (1.12–1.26) | <0.001 | ||||
| Patients with stage | ||||||
| PCS | ||||||
| ≥45.7 | 282 | 64 | 1.00 (Ref) | 16.6 | ||
| 32.7–45.7 | 305 | 32 | 1.32 (1.12–1.57) | 0.001 | 12.5 | |
| <32.7 | 311 | 19 | 2.05 (1.71–2.45) | <0.001 | 8.5 | <0.001 |
| P for trend | 1.43 (1.31–1.57) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 282 | 53 | 1.00 (Ref) | 13.7 | ||
| 40.3–52.3 | 295 | 32 | 1.10 (0.92–1.30) | 0.30 | 12.6 | |
| <40.3 | 321 | 30 | 1.40 (1.19–1.66) | <0.001 | 10.4 | <0.001 |
| P for trend | 1.19 (1.09–1.29) | <0.001 | ||||
| Stage (I, II) | ||||||
| PCS | ||||||
| ≥45.7 | 107 | 41 | 1.00 (Ref) | 24.3 | ||
| 32.7–45.7 | 82 | 17 | 1.56 (1.14–2.12) | 0.005 | 18.6 | |
| <32.7 | 62 | 9 | 1.78 (1.27–2.51) | <0.001 | 14.0 | <0.001 |
| P for trend | 1.35 (1.15–1.60) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 98 | 33 | 1.00 (Ref) | 22.6 | ||
| 40.3–52.3 | 70 | 18 | 0.94 (0.67–1.32) | 0.72 | 21.1 | |
| <40.3 | 83 | 16 | 1.39 (1.00–1.92) | 0.049 | 15.5 | 0.26 |
| P for trend | 1.18 (1.00–1.40) | 0.05 | ||||
| Stage (III, IV) | ||||||
| PCS | ||||||
| ≥45.7 | 175 | 23 | 1.00 (Ref) | 13.7 | ||
| 32.7–45.7 | 223 | 15 | 1.28 (1.04–1.58) | 0.02 | 10.0 | |
| <32.7 | 249 | 10 | 2.15 (1.72–2.69) | <0.001 | 7.2 | <0.001 |
| P for trend | 1.47 (1.32–1.65) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 184 | 20 | 1.00 (Ref) | 10.1 | ||
| 40.3–52.3 | 225 | 14 | 1.14 (0.93–1.40) | 0.22 | 9.9 | |
| <40.3 | 238 | 14 | 1.44 (1.18–1.77) | <0.001 | 8.4 | 0.01 |
| P for trend | 1.20 (1.09–1.33) | <0.001 | ||||
| Whites | ||||||
| PCS | ||||||
| ≥45.7 | 490 | 188 | 16.6 | |||
| 32.7–45.7 | 531 | 124 | 1.38 (1.21–1.56) | <0.001 | 12.9 | |
| <32.7 | 527 | 106 | 2.00 (1.75–2.29) | <0.001 | 9.2 | <0.001 |
| P for trend | 1.41 (1.32–1.51) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 496 | 160 | 15.1 | |||
| 40.3–52.3 | 535 | 135 | 1.25 (1.10–1.41) | <0.001 | 12.8 | |
| <40.3 | 517 | 123 | 1.42 (1.25–1.61) | <0.001 | 10.3 | <0.001 |
| P for trend | 1.19 (1.12–1.27) | <0.001 | ||||
| African-Americans | ||||||
| PCS | ||||||
| ≥45.7 | 25 | 9 | 1.00 (Ref) | 14.4 | ||
| 32.7–45.7 | 45 | 9 | 2.02 (1.06–3.86) | 0.03 | 12.4 | |
| <32.7 | 65 | 13 | 3.08 (1.63–5.79) | <0.001 | 7.9 | 0.01 |
| P for trend | 1.71 (1.27–2.31) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 35 | 8 | 1.00 (Ref) | 10.0 | ||
| 40.3–52.3 | 41 | 11 | 0.80 (0.43–1.49) | 0.48 | 10.2 | |
| <40.3 | 59 | 12 | 1.25 (0.70–2.23) | 0.45 | 8.6 | 0.45 |
| P for trend | 1.15 (0.86–1.55) | 0.34 | ||||
| Hispanics | ||||||
| PCS | ||||||
| ≥45.7 | 20 | 14 | 17.6 | |||
| 32.7–45.7 | 63 | 9 | 1.18 (0.65–2.17) | 0.58 | 11.8 | |
| <32.7 | 66 | 14 | 1.77 (0.96–3.26) | 0.07 | 9.3 | 0.003 |
| P for trend | 1.37 (1.03–1.83) | 0.03 | ||||
| MCS | ||||||
| ≥52.3 | 27 | 13 | 13.2 | |||
| 40.3–52.3 | 50 | 12 | 1.24 (0.70–2.21) | 0.46 | 11.0 | |
| < 40.3 | 72 | 12 | 1.27 (0.72–2.23) | 0.41 | 11.3 | 0.17 |
| P for trend | 1.10 (0.85–1.44) | 0.47 | ||||
| African-American/Hispanics/others | ||||||
| PCS | ||||||
| ≥45.7 | 72 | 42 | 17.1 | |||
| 32.7–45.7 | 154 | 33 | 1.47 (1.07–2.03) | 0.02 | 12.4 | |
| <32.7 | 167 | 44 | 2.00 (1.45–2.76) | <0.001 | 9.4 | <0.001 |
| P for trend | 1.41 (1.20–1.64) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 98 | 41 | 13.7 | |||
| 40.3–52.3 | 129 | 47 | 1.27 (0.93–1.72) | 0.13 | 11.0 | |
| <40.3 | 166 | 31 | 1.45 (1.08–1.94) | 0.01 | 11.1 | 0.007 |
| P for trend | 1.20 (1.04–1.38) | 0.02 | ||||
| No treatment before survey | ||||||
| PCS | ||||||
| ≥45.7 | 464 | 167 | 1.00 (Ref) | 15.7 | ||
| 32.7–45.7 | 516 | 102 | 1.35 (1.18–1.53) | <0.001 | 11.6 | |
| <32.7 | 547 | 105 | 1.93 (1.69–2.21) | <0.001 | 8.3 | <0.001 |
| P for trend | 1.39 (1.30–1.49) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 453 | 130 | 1.00 (Ref) | 13.0 | ||
| 40.3–52.3 | 523 | 131 | 1.19 (1.04–1.35) | <0.001 | 11.6 | |
| <40.3 | 551 | 113 | 1.34 (1.18–1.53) | <0.001 | 9.9 | <0.001 |
| P for trend | 1.16 (1.09–1.23) | <0.001 | ||||
| Treatment before survey | ||||||
| PCS | ||||||
| ≥45.7 | 98 | 63 | 1.00 (Ref) | 19.8 | ||
| 32.7–45.7 | 169 | 55 | 1.53 (1.17–2.00) | 0.002 | 17.0 | |
| <32.7 | 147 | 45 | 2.16 (1.64–2.85) | <0.001 | 12.7 | <0.001 |
| P for trend | 1.47 (1.28–1.68) | <0.001 | ||||
| MCS | ||||||
| ≥52.3 | 141 | 71 | 1.00 (Ref) | 19.3 | ||
| 40.3–52.3 | 141 | 51 | 1.63 (1.26–2.11) | <0.001 | 15.1 | |
| < 40.3 | 132 | 41 | 1.91 (1.46–2.51) | <0.001 | 13.5 | <0.001 |
| P for trend | 1.39 (1.21–1.58) | <0.001 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio; MST, medium survival time; MCS, Mental Component Summary; PCS, Physical Component Summary.
Adjusted for sex, age, marital status, race, education level, occupation, smoking, alcohol use, tumour size, cancer stage, comorbidity, treatment before survey, time since diagnosis and years of diagnosis.
Fig. 1.
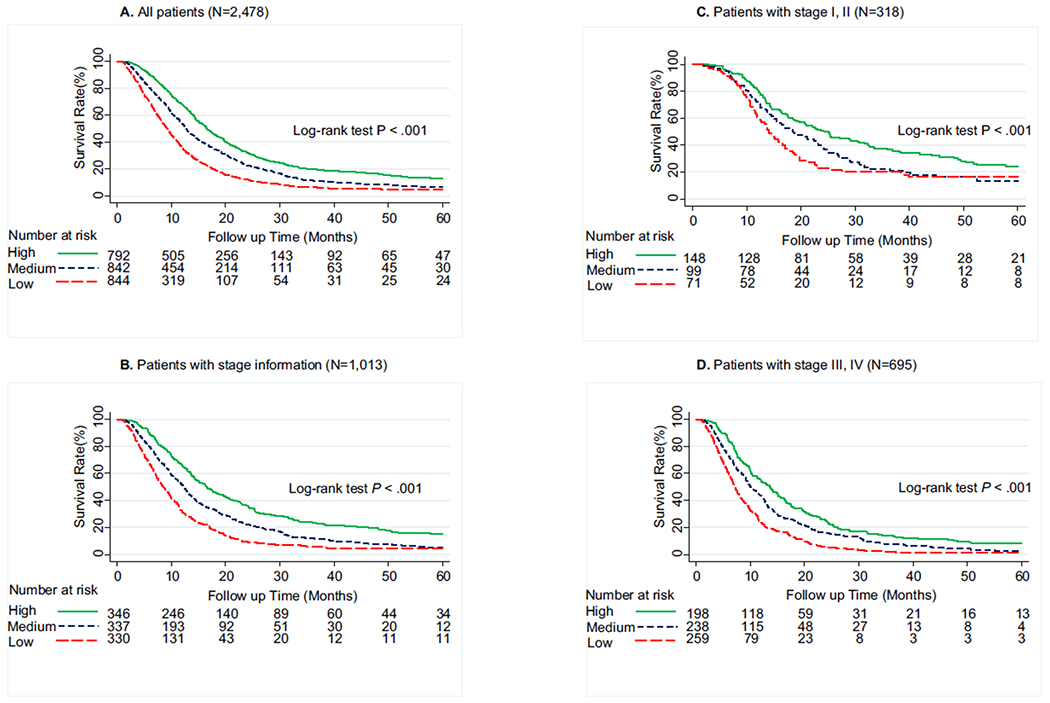
Five-year survival of pancreatic ductal adenocarcinoma cancer patients by Physical Component Summary (PCS) scores categorised into tertiles. (A) Overall population (N = 2478), (B) patients with available tumour stage information (N = 1013), (C) patients with stage I & II (N = 318), (D) patients with stage III & IV (N = 695). Higher PCS scores indicate better physical quality of life. High, ≥45.7; medium, 32.7–45.7; low, <32.7.
Fig. 2.
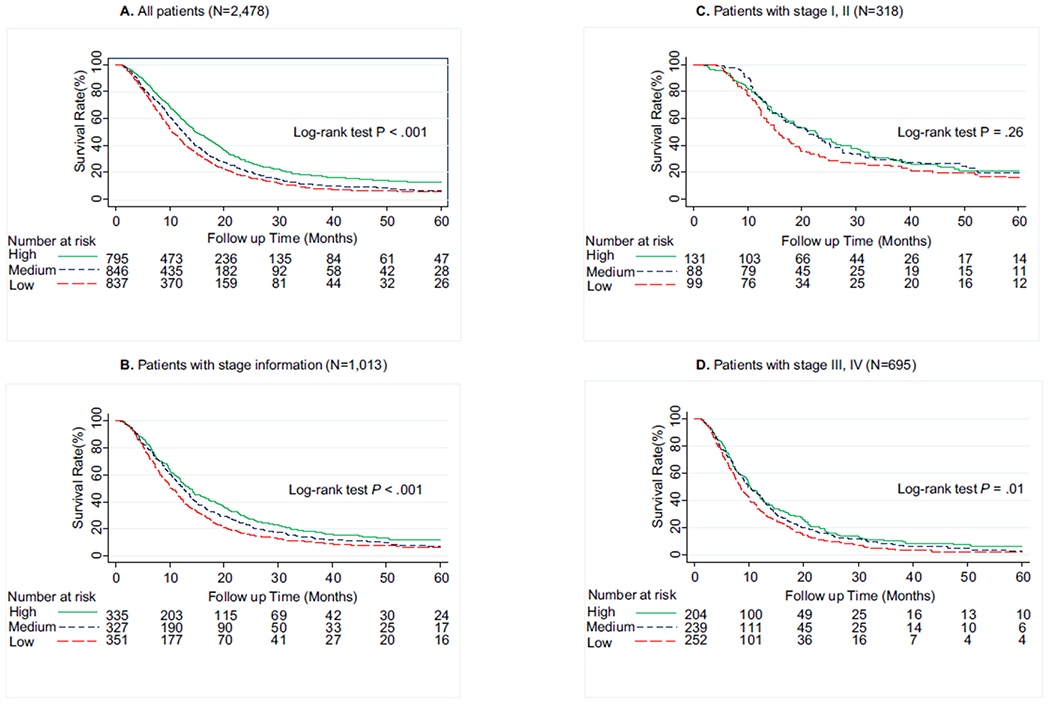
Five-year survival of pancreatic ductal adenocarcinoma cancer patients by Mental Component Summary (MCS) scores categorised into tertiles. (A) Overall population (N = 2478), (B) patients with available tumour stage information (N = 1013), (C) patients with stage I & II (N = 318), (D) patients with stage III & IV (N = 695). Higher MCS scores indicate better mental quality of life. High, ≥52.3; medium, 40.3–52.3; low, <40.3.
4. Discussion
In this study, we evaluated the association of QOL after diagnosis with survival and explored the determinants of QOL in PDAC patients. Two main findings were obtained. First, QOL after diagnosis was a significant prognostic factor for overall survival. Second, multiple sociodemographic and clinical factors affected QOL. To the best of our knowledge, this is the first study using the SF-12v1 questionnaire to probe the prognostic value and the determinants of QOL in a large cohort of racially/ ethnically diverse patients with PDAC.
Consistent with results from previous studies [5–13], our study demonstrated that better QOL was significantly associated with longer survival time in patients with PDAC. Furthermore, this effect on survival was consistent across different racial/ethnic groups. The mechanism by which QOL affects survival is not completely understood. The first possible mechanism is related to elevated inflammatory activation. Elevated inflammatory activation is observed in patients who have poor QOL [20,21] and also has been found in PDAC patients with poor survival [22,23]. Therefore, dysregulation of some pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) may explain the relationship between QOL and survival in PDAC patients. The second possible mechanism is associated with the patient’s stress [24]. A review of studies of animal models and humans indicated chronic stress and depression impair the immune response and may promote the initiation and progression of some types of cancer [25]. In addition, another animal study also shows under chronic stress, dopamine (DA) levels in brain are lower as a consequence of decreased release of DA [26], which has been demonstrated to inhibit tumour growth via the activation of dopamine receptor D2 (DRD2) [27]. Therefore, poor QOL with weaken immune responses and low level of DA may contribute to tumour progression and ultimately influence PDAC patients’ overall survival. The third possible mechanism is related to the patient’s physical ability to tolerate treatment. A clinical trial demonstrated that a lower physical well-being score was related to worse response to treatment and shorter survival duration in patients with lung cancer [28]. In addition, QOL could influence the treatment decision-making for PDAC patients [16]. Interestingly, we found no significant prognostic value of low MCS for stage I, II PDAC. Although we have adjusted many potential confounding factors and performed stratified analysis by cancer stage, race/ethnicity, and treatment before survey to minimise the impact from these factors, we could not exclude the possibility of residual confounding from unmeasured common factors. Further studies need to explore the underlying mechanisms. Our findings suggest QOL measures may provide clinicians with helpful information on the monitoring and treatment of PDAC patients.
Our study also identified multiple determinants of physical and mental QOL and most of these determinants similarly influenced QOL across the different racial/ethnic groups. We found Hispanic patients had lower mean PCS and MCS scores than non-Hispanic whites. Previous studies also indicated Hispanic cancer patients experience lower QOL [29]. Socioeconomic status (SES) appears to be the main reason for this disparity. SES has been shown to be related to race/ethnicity. More minority than white residents of the United States are in low SES categories [30]. Low SES can influence access to medical care and is related to higher rates of comorbidities and later disease stage at diagnosis in minority populations [30,31]. Our findings suggest that Hispanic PDAC patients are at increased risk of lower QOL and appropriate supportive interventions should be formulated for this group of patients.
We found women and younger patients were more likely to report poor mental QOL than were men and elderly patients, which suggests sex and age should be considered in clinical practice. One recent study showed sex is an important predictive factor for QOL and women with cancer had poorer QOL than men [32]. One possible reason is that somatic symptoms influence quality of life more deleteriously among women than men [33]. One recent study showed that some QOL components (social functioning and financial problems) improve with age, whereas other components (physical functioning and constipation) deteriorate with age in cancer patients [34]. Interestingly, our study showed elderly patients had better mental QOL than younger patients. This may be due to older adults having more adaptive experience of severe illness [35] and bearing less of a financial burden [34].
Our results showed tumour stage is an independent factor that predicts physical QOL in PDAC patients. This finding was consistent with the results from one recent study of pancreatic cancer [36]. Advanced tumours tend to infiltrate the retroperitoneal nerve plexus, bile duct, stomach, and duodenum, causing abdominal and mid-back pain, obstructive jaundice, vomiting, mal-digestion, and cachexia [37]. All of these symptoms negatively affect the QOL of PDAC patients. This study indicates clinicians should focus on interventions to alleviate the symptom burden of advanced PDAC patients.
A notable finding of our study is that the time period of one to three months from diagnosis was a risk factor of low PCS. Longitudinal assessment of QOL during diagnosis and treatment of PC is of great interest. Previous studies on surgery showed that pancreatectomy had a short-term negative impact on patient’s QOL within 3 months [38–40], whereas QOL recovered from surgery after 6 months [15,39,40]. Several studies among patients on chemotherapy reported an improvement of QOL after chemotherapy compared with baseline [11,41,42]. Specifically, a previous study found that QOL improved at the end of treatment (6 months) among patients on the FOLFIRINOX chemotherapy regimen [11]. In another study among patients treated by gemcitabine or gemcitabine combined with capecitabine, an improvement in mood and coping effort was noted in both groups within 2–5 months after starting treatment [41]. In a third study, global QOL was significantly improved after receiving fluorouracil combined with mitomycin for 6 months [42]. Two studies also found that the improvement in QOL of cognitive function within 3 months [9] and physical function at 2 months [12] predicted improved survival. Another concern of researchers during the longitudinal assessment of QOL is response shift of cancer patients. Cancer patients are faced with the necessity to adapt to their illness. Response shift is an important mediator of this adaption, which involves the change of internal standards, values and conceptualisation of QOL [43]. Integrating response shift into QOL assessment allows researchers to better understand the longitudinal change of QOL in cancer patients, which requires more extensive research.
The 5-year survival of PC patients has improved over the past several decades, from 3.0% in 1975 to 8.5% now [44]. Our study showed a trend of increasing PCS and MCS from 1999 to 2012, which we hypothesised was representative of the advancement in the treatment and medical care of PDAC. Given the potential positive impact of favourable QOL on improving survival of PDAC patients and understanding the determinants of QOL, we can expect further improvement of survival of PDAC by targeting the determinants of QOL in the future.
A major strength of our study is the large, diverse PDAC patient population. Our findings can be generalised to both non-Hispanic whites and other racial/ethnic groups. Second, patients with localised (I, II) disease were included, whereas other studies only focused on patients with metastatic or advanced stage [6,9–12]. Third, the SF-12v1 questionnaire is easy and reliable to use in routine clinical practice [45] and can assess physical and mental QOL separately. The main limitation to our study is that tumour stage information was missing for 1465 of the 2478 patients, however, our sensitivity analysis showed similar results when limiting the analysis to patients with tumour stage information. In addition, education and occupation were used as indicators of social class, but information on other social class indicators (e.g. family income) was not available. Finally, we did not perform the longitudinal assessment of QOL and could not investigate whether changes in QOL during treatment could predict survival of patients with PC.
In summary, this study highlighted that QOL after diagnosis is an independent prognostic indicator for PDAC. QOL measurement could help clinicians identify subpopulations of PDAC patients who are at risk of poor survival, which may be helpful in monitoring patients or formulating interventions. We also identified multiple sociodemographic and clinical factors that can influence the QOL of PDAC patients. Clinicians could use these factors to tailor individualised interventions aimed at improving QOL and survival in PDAC patients.
Supplementary Material
Acknowledgements
The authors thank the epidemiologists and field workers of Department of Epidemiology at The University of Texas MD Anderson Cancer Center for their excellent work on the data collection for this study.
Funding
This work was supported by the funds collected pursuant to the Comprehensive Tobacco Settlement to the University of Texas MD Anderson Cancer Center. Additional funding was provided by the Center for Translational and Public Health Genomics, Dan Duncan Family Institute for Risk Assessment and Cancer Prevention and MD Anderson’s Cancer Center Support Grant from the National Cancer Institute at the National Institutes of Health [P30, CA016672]. Dr. Klein is supported by the National Cancer Institute at the National Institutes of Health [P50, CA062924].
Footnotes
Conflict of interest statement
None declared.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejca.2017.12.023.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- [3].American Cancer Society. Cancer facts and figures 2017. 2017. [acessed 08.05.17], https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.
- [4].Muller-Nordhorn J, Roll S, Bohmig M, Nocon M, Reich A, Braun C, et al. Health-related quality of life in patients with pancreatic cancer. Digestion 2006;74(2):118–25. [DOI] [PubMed] [Google Scholar]
- [5].Velanovich V The association of quality-of-life measures with malignancy and survival in patients with pancreatic pathology. Pancreas 2011;40(7):1063–9. [DOI] [PubMed] [Google Scholar]
- [6].Diouf M, Filleron T, Pointet AL, Dupont-Gossard AC, Malka D, Artru P, et al. Prognostic value of health-related quality of life in patients with metastatic pancreatic adenocarcinoma: a random forest methodology. Qual Life Res 2016;25(7):1713–23. [DOI] [PubMed] [Google Scholar]
- [7].Quinten C, Martinelli F, Coens C, Sprangers MA, Ringash J, Gotay C, et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2014;120(2):302–11. [DOI] [PubMed] [Google Scholar]
- [8].Lis CG, Gupta D, Grutsch JF. Patient satisfaction with quality of life as a predictor of survival in pancreatic cancer. Int J Gastrointest Cancer 2006;37(1):35–44. [DOI] [PubMed] [Google Scholar]
- [9].Braun DP, Gupta D, Staren ED. Longitudinal health-related quality of life assessment implications for prognosis in stage IV pancreatic cancer. Pancreas 2013;42(2):254–9. [DOI] [PubMed] [Google Scholar]
- [10].Bernhard J, Dietrich D, Glimelius B, Hess V, Bodoky G, Scheithauer W, et al. Estimating prognosis and palliation based on tumour marker CA 19-9 and quality of life indicators in patients with advanced pancreatic cancer receiving chemotherapy. Br J Cancer 2010;103(9):1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouche O, Guimbaud R, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31(1):23–9. [DOI] [PubMed] [Google Scholar]
- [12].Vickers MM, Lee C, Tu D, Wheatley-Price P, Parulekar W, Brundage MD, et al. Significance of baseline and change in quality of life scores in predicting clinical outcomes in an international phase III trial of advanced pancreatic cancer: NCIC CTG PA.3. Pancreatology 2016;16(6):1106–12. [DOI] [PubMed] [Google Scholar]
- [13].Sugimoto H, Kawashima H, Ohno E, Hayashi D, Kuwahara T, Morishima T, et al. The prognostic factors and trajectory of HRQOL in patients with pancreatic cancer who received psychiatric intervention. J Gastroenterol Hepatol 2016;31(3): 685–90. [DOI] [PubMed] [Google Scholar]
- [14].Anwar S, Tan W, Yu J, Hutson A, Javle M, Iyer R. Quality-of-life (QoL) as a predictive biomarker in patients with advanced pancreatic cancer (APC) receiving chemotherapy: results from a prospective multicenter phase 2 trial. J Gastrointest Oncol 2014; 5(6):433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park JW, Jang JY, Kim EJ, Kang MJ, Kwon W, Chang YR, et al. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. Br J Surg 2013;100(8):1064–70. [DOI] [PubMed] [Google Scholar]
- [16].Crippa S, Dominguez I, Rodriguez JR, Razo O, Thayer SP, Ryan DP, et al. Quality of life in pancreatic cancer: analysis by stage and treatment. J Gastrointest Surg 2008;12(5):783–93. discussion 793-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Davidson W, Ash S, Capra S, Bauer J. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr 2004;23(2):239–47. [DOI] [PubMed] [Google Scholar]
- [18].Wu X, Hildebrandt MA, Ye Y, Chow WH, Gu J, Cunningham S, et al. Cohort profile: the MD Anderson Cancer Patients and Survivors Cohort (MDA-CPSC). Int J Epidemiol 2016;45(3). 713–713f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- [20].Mommersteeg PM, Kupper N, Schoormans D, Emons W, Pedersen SS. Health-related quality of life is related to cytokine levels at 12 months in patients with chronic heart failure. Brain Behav Immun 2010;24(4):615–22. [DOI] [PubMed] [Google Scholar]
- [21].Parissis JT, Nikolaou M, Farmakis D, Paraskevaidis IA, Bistola V, Venetsanou K, et al. Self-assessment of health status is associated with inflammatory activation and predicts long-term outcomes in chronic heart failure. Eur J Heart Fail 2009;11(2): 163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gasiorowska A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Malecka-Panas E. Subclinical inflammation and endothelial dysfunction in patients with chronic pancreatitis and newly diagnosed pancreatic cancer. Dig Dis Sci 2016;61(4):1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim HW, Lee JC, Paik KH, Kang J, Kim J, Hwang JH. Serum interleukin-6 is associated with pancreatic ductal adenocarcinoma progression pattern. Medicine (Baltimore) 2017;96(5):e5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- [25].Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004;5(10):617–25. [DOI] [PubMed] [Google Scholar]
- [26].Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res 1992;577(2):194–9. [DOI] [PubMed] [Google Scholar]
- [27].Peters MA, Walenkamp AM, Kema IP, Meijer C, de Vries EG, Oosting SF. Dopamine and serotonin regulate tumor behavior by affecting angiogenesis. Drug Resist Updates 2014;17(4–6):96–104. [DOI] [PubMed] [Google Scholar]
- [28].Eton DT, Fairclough DL, Cella D, Yount SE, Bonomi P, Johnson DH. Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol 2003;21(8):1536–43. [DOI] [PubMed] [Google Scholar]
- [29].Movsas B, Scott C, Watkins-Bruner D. Pretreatment factors significantly influence quality of life in cancer patients: a Radiation Therapy Oncology Group (RTOG) analysis. Int J Radiat Oncol Biol Phys 2006;65(3):830–5. [DOI] [PubMed] [Google Scholar]
- [30].Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer 2008;113(3):582–91. [DOI] [PubMed] [Google Scholar]
- [31].Scheppers E, van Dongen E, Dekker J, Geertzen J, Dekker J. Potential barriers to the use of health services among ethnic minorities: a review. Fam Pract 2006;23(3):325–48. [DOI] [PubMed] [Google Scholar]
- [32].Geue K, Sender A, Schmidt R, Richter D, Hinz A, Schulte T, et al. Gender-specific quality of life after cancer in young adulthood: a comparison with the general population. Qual Life Res 2014;23(4):1377–86. [DOI] [PubMed] [Google Scholar]
- [33].Larsson M, Ljung L, Johansson BB. Health-related quality of life in advanced non-small cell lung cancer: correlates and comparisons to normative data. Eur J Cancer Care (Engl) 2012;21(5): 642–9. [DOI] [PubMed] [Google Scholar]
- [34].Quinten C, Coens C, Ghislain I, Zikos E, Sprangers MA, Ringash J, et al. The effects of age on health-related quality of life in cancer populations: a pooled analysis of randomized controlled trials using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur J Cancer 2015;51(18):2808–19. [DOI] [PubMed] [Google Scholar]
- [35].Piazza JR, Charles ST, Almeida DM. Living with chronic health conditions: age differences in affective well-being. J Gerontol B Psychol Sci Soc Sci 2007;62(6):P313–21. [DOI] [PubMed] [Google Scholar]
- [36].Moningi S, Walker AJ, Hsu CC, Reese JB, Wang JY, Fan KY, et al. Correlation of clinical stage and performance status with quality of life in patients seen in a pancreas multidisciplinary clinic. J Oncol Pract 2015;11(2):e216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011;378(9791):607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Eaton AA, Gonen M, Karanicolas P, Jarnagin WR, D’Angelica MI, DeMatteo R, et al. Health-related quality of life after pancreatectomy: results from a randomized controlled trial. Ann Surg Oncol 2016;23(7):2137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rees JR, Macefield RC, Blencowe NS, Alderson D, Finch-Jones MD, Blazeby JM. A prospective study of patient reported outcomes in pancreatic and peri-ampullary malignancy. World J Surg 2013;37(10):2443–53. [DOI] [PubMed] [Google Scholar]
- [40].Chan C, Franssen B, Dominguez I, Ramirez-Del Val A, Uscanga LF, Campuzano M. Impact on quality of life after pancreatoduodenectomy: a prospective study comparing preoperative and postoperative scores. J Gastrointest Surg 2012;16(7): 1341–6. [DOI] [PubMed] [Google Scholar]
- [41].Bernhard J, Dietrich D, Scheithauer W, Gerber D, Bodoky G, Ruhstaller T, et al. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial—SAKK 44/00-CECOG/PAN.1.3.001. J Clin Oncol 2008;26(22):3695–701. [DOI] [PubMed] [Google Scholar]
- [42].Maisey N, Chau I, Cunningham D, Norman A, Seymour M, Hickish T, et al. Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5-FU) with PVI 5-FU plus mitomycin in inoperable pancreatic cancer. J Clin Oncol 2002;20(14):3130–6. [DOI] [PubMed] [Google Scholar]
- [43].Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med 1999;48(11):1507–15. [DOI] [PubMed] [Google Scholar]
- [44].National Cancer Institute surveillance, epidemiology, and end results program. Cancer stat facts: pancreas cancer. 2017. [accessed 19.07.17], https://seer.cancer.gov/statfacts/html/pancreas.html. [Google Scholar]
- [45].Pezzilli R, Morselli-Labate AM, Fantini L, Campana D, Corinaldesi R. Assessment of the quality of life in chronic pancreatitis using Sf-12 and EORTC Qlq-C30 questionnaires. Dig Liver Dis 2007;39(12):1077–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


