Figure 2.
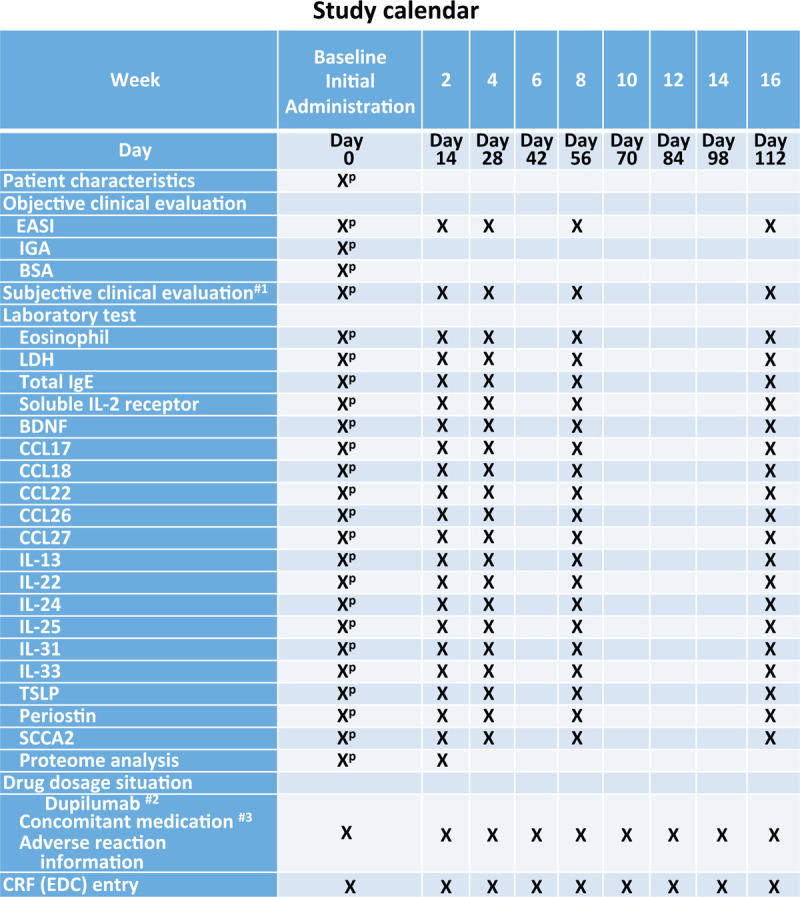
Study calendar is depicted. 1. Subjective clinical evaluation includes POEM, Pruritus-NRS, Uncomfortable skin-NRS, and Treatment satisfaction-NRS. 2. The administration of dupilumab shall be carried out after all assessments and tests are completed. A change of administration day is allowed within the range of +/- 1 week. #3. Use of ocular, intranasal, or inhalant steroids, calcineurin inhibitors, and antihistamines is allowed throughout the study. P = pre-treatment.
