Abstract
Background
Health departments utilize HIV surveillance data to identify people with HIV (PWH) who need re-linkage to HIV care as part of an approach known as Data to Care (D2C.) The most accurate, effective, and efficient method of identifying PWH for re-linkage is unknown.
Methods
We evaluated referral and care continuum outcomes among PWH identified using 3 D2C referral strategies: health care providers, surveillance, and a combination list derived by matching an electronic medical record registry to HIV surveillance. PWH who were enrolled in the re-linkage intervention received short-term case management for up to 90 days. Relative risks and 95% confidence intervals were calculated to compare proportions of PWH retained and virally suppressed before and after re-linkage. Durable viral suppression was defined as having suppressed viral loads at all viral load measurements in the 12 months after re-linkage.
Results
After initial investigation, 233 (24%) of 954 referrals were located and enrolled in navigation. Although the numbers of surveillance and provider referrals were similar, 72% of enrolled PWH were identified by providers, 16% by surveillance, and 12% by combination list. Overall, retention and viral suppression improved, although relative increases in retention and viral suppression were only significant among individuals identified by surveillance or providers. Seventy percent of PWH who achieved viral suppression after the intervention remained durably virally suppressed.
Conclusions
PWH referred by providers were more likely to be located and enrolled in navigation than PWH identified by surveillance or combination lists. Overall, D2C re-linkage efforts improved retention, viral suppression, and durable viral suppression.
Keywords: Data to Care, HIV, linkage to care, retention, surveillance
In San Francisco, the goal of “Ending the HIV Epidemic” appears achievable [1]. From 2013 to 2018, the number of new HIV diagnoses in San Francisco decreased by 51%, from 399 to 197 [2]. During this time period, linkage-to-care efforts intensified to ensure that newly diagnosed patients were linked to care as rapidly as possible, and linkage to care in 30 days among new diagnoses improved from 74% in 2012 to 91% in 2018 [2]. Despite this impressive decline in new diagnoses and decreases in time to HIV care, the percentage of all people with HIV (PWH) who are virally suppressed increased only from 66% in 2013 to 74% in 2017 [3]. In 2017, viral suppression lagged for African Americans (68%), Latinx individuals (70%), women (66%), trans women (68%), people who inject drugs (PWID; 65%), and people experiencing homelessness (33%) [3].
Improving viral suppression across all populations is critical to reducing disparities and getting to 0 new infections. An estimated 43% of new HIV infections are attributed to people who are aware of their HIV-positive status but are not engaged in medical care [4]. Since 2012, federal funding has allowed some local health departments to expand their partner services programs, which traditionally focused on linking newly diagnosed PWH to care, to also offer HIV re-linkage services for PWH who are not in care (NIC). In response, the San Francisco Department of Public Health (SFDPH) developed the Linkage, Integration, Navigation and Comprehensive Services (LINCS) team to locate NIC PWH, identify barriers to care, and re-link them to care through short-term case management [5].
The Centers for Disease Control and Prevention (CDC) encourages health department prevention programs to work closely with local HIV surveillance as part of a public health strategy called Data to Care (D2C) [6, 7]. The goal of D2C is to identify people who are NIC based on the absence of reportable HIV-related lab values such as viral load and CD4 cell count and assign them to an HIV navigator, who then works to locate them, offer services, and assist with linkage to HIV care [8]. Implementation of D2C varies across jurisdictions, and published reports describe successful re-linkage outcomes from 3 main D2C referral sources: (1) health care providers [9–11], (2) HIV surveillance [12–16], and (3) a combination approach in which a list of patients lost to follow-up from an HIV care clinic is matched to HIV surveillance [3, 6, 17–19]. Several jurisdictions cite inefficiencies of surveillance-only-generated referrals because of the overidentification of “current to care” PWH who appear to be NIC by surveillance because of delayed lab reporting or relocation across state lines [11, 13, 14, 16, 20, 21].
Two of the 4 pillars of the federal End the HIV Epidemic plan hinge on robust health department–based HIV re-linkage programs in order to increase HIV viral suppression (“Treat”) and respond to HIV outbreaks (“Respond”) [1]; however, the efficiency and effectiveness of D2C referral strategies are unknown [17, 22]. From 2015 to 2017, the SFDPH LINCS navigation program utilized 3 referral strategies in parallel to identify and re-link NIC PWH. The objective of this study was to compare key outcomes of each strategy, including the proportion of PWH who were (1) able to be located and enrolled by staff, (2) re-engaged in HIV care, (3) virally suppressed 12 months after enrollment, and (4) durably virally suppressed. We compared retention and viral suppression 12 months before and after the LINCS intervention and analyzed outcomes across key subgroups in which we are working to reduce HIV health disparities.
METHODS
Setting
LINCS navigators were initially based at the health department’s municipal sexually transmitted disease clinic, along with other disease intervention specialists who provide syphilis and HIV partner services. HIV care providers at large public health clinics referred PWH whom they suspected had fallen out of care to LINCS. In 2015, the SFDPH received funding to hire additional LINCS navigators, who were embedded in 3 public health clinics. With expanded resources, LINCS and HIV surveillance collaborated to develop and implement a citywide D2C program using surveillance data to identify NIC PWH for re-linkage.
Identification of Persons Not in Care
Between 2015 and 2017, LINCS received referrals from (1) health care providers, (2) HIV surveillance, and (3) a clinical electronic medical record (EMR) registry matched to surveillance. All referrals were identified through the absence of viral load or CD4 test results or the presence of a recent high viral load value (eg, VL >1500 copies/mL within last 4 months or no VL in >15 months). HIV surveillance epidemiologists used these viral load or CD4 criteria to identify patients in the Enhanced HIV/AIDS Reporting System (eHARS) for referral. Provider referrals also included patients who had no evidence of care postdiagnosis, did not access care over many months, or who were not adherent to medication. Given known disparities in viral suppression, the initial surveillance lists were of black and Latinx NIC PWH who were last known to reside in San Francisco per the Enhanced HIV/AIDS Reporting System, the state HIV surveillance system. The clinical EMR match was conducted utilizing a registry developed through Health Resources and Services Administration HIV Ryan White quality improvement activities. The registry included all PWH receiving care in the 3 public health clinics where LINCS navigators were embedded. This registry was matched to eHARS in order to exclude persons who did not meet our inclusion criteria, had moved outside San Francisco, died, or had been assigned to LINCS in the past year. The final matched lists were sent to LINCS following SFDPH data security protocols. LINCS staff checked eHARS to determine current not-in-care status for referrals from all 3 sources before starting outreach. Not in care was defined as VL >1500 copies/mL within the last 4 months or no VL in >15 months, per eHARS.
Initial Investigation and Re-linkage Services
Navigators attempted to locate individuals within 30 days of assignment using access to multiple electronic systems including the local STD surveillance database, the public health hospital EMR, and other disease intervention searching tools. Referral outcomes include (1) enrollment in LINCS, (2) refusal of LINCS services (either by directly refusing or by failing to follow up with the navigator after initial contact), (3) already in care based on record review or patient self-report, (4) not located despite multiple attempts, and (5) ineligible (moved outside San Francisco, already in case management, incarcerated, deceased, or severe medical or psychiatric barriers that prevented outreach). If PWH were found to be deceased or to have relocated out of jurisdiction, that information was transmitted back to HIV surveillance.
Enrollment in LINCS required that a PWH attend a re-linkage appointment with an HIV care provider. Navigators worked with PWH for up to 90 days and would offer a warm hand-off to a long-term case manager for ongoing support as needed. Typical caseloads ranged from 15–25 PWH at a time. Navigators provided a range of field-based services to address barriers to care, including benefits navigation, appointment reminders, accompaniment to clinic, motivational interviewing, and modified Anti-Retroviral Treatment and Access to Services strengths-based case management (Supplementary Data).
Analysis
Referral and enrollment dates and outcomes, drug use, and housing status were collected and entered into the STD database by navigators. Homelessness was defined using the US Department of Housing and Urban Development definition and included individuals who were “doubled up” in the homes of family or friends, homeless upon leaving jails, hospitals, and rehabilitation facilities, families living in single-room occupancy (SRO) units, and individuals living in SRO units <30 days. For PWH with >1 navigation assignment during the time period (~10% of all referrals), we included the outcome from the last assignment in the implementation period.
All other variables, including demographic and clinical care outcomes, were obtained through eHARS. For each individual referred to LINCS, we used eHARS to determine (1) retention in HIV care, (2) viral suppression, and (3) durable viral suppression. Retention was defined as having 2 tests (viral load, CD4, or genotype) at least 90 days apart in the 12 months before LINCS enrollment (pre-LINCS retention) and in the 12 months after assignment closure date (post-LINCS retention). Viral suppression was defined as having at least 1 viral load <200 copies/mL at any time within the 12 months before LINCS enrollment or after LINCS closure. Ever virally suppressed was any viral load <200 copies/mL at any time before LINCS enrollment. Durable viral suppression was computed postenrollment for PWH who achieved viral suppression and was defined as having all viral loads suppressed after the first suppressed viral load. HIV-related lab data were extracted as of February 2019 from eHARS. PWH without 12 months of follow-up time were excluded from the durable viral suppression analysis. Relative risks (RRs) and 95% CIs were calculated to compare pre- and post-LINCS proportions for retention and viral suppression and stratified based on age, gender, race, housing status, drug use, and past evidence of viral suppression. All analyses were conducted using SAS 9.4 (Cary, NC, USA).
Patient Consent Statement
This is a secondary analysis of data routinely collected by the SFDPH, and no institutional review board approval or consent was necessary.
RESULTS
Outcomes From Initial Investigation of Referred PWH
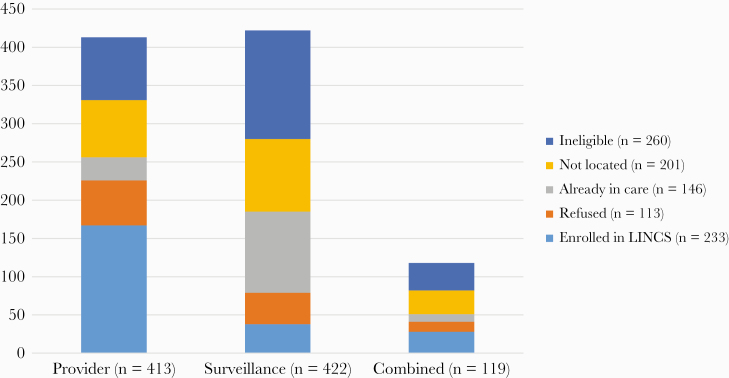
From 2015 to 2017, 954 patients were referred for LINCS navigation from 3 referral sources: 43% by providers, 44% by surveillance, and 13% by electronic medical record and surveillance combination match list. Demographic and clinical characteristics of the patients referred, stratified by referral source, are provided in the Supplementary Data. PWH identified and referred by providers were more likely to be enrolled in LINCS (167/413; 40%) compared with those referred from surveillance (38/422; 9%) or combination match list (28/119; 24%). A greater proportion of surveillance-generated referrals were ineligible compared with provider and combination referrals (34% compared with 20% and 30%, respectively) or already in care (25% compared with 7.2% and 8.4%, respectively). After initial contact, 14.2% of provider, 9.7% of surveillance, and 11.0% of combination referrals declined to enroll in LINCS (Figure 1).
Figure 1. .
Outcomes from initial investigation of referred people with HIV by referral source. Provider-identified referrals were most likely to be located and enrolled in navigation. Abbreviation: LINCS, Linkage, Integration, Navigation and Comprehensive Services.
Characteristics of Patients Located and Enrolled in Navigation
Among 233 PWH enrolled in navigation from 2015 to 2017, 72% were identified by providers, 16% through surveillance and 12% from combination match lists (Table 1). A greater proportion of patients referred by providers were experiencing homelessness compared with surveillance and combination match referrals (53% compared with 21% and 29%, respectively; P < .001) and were classified as men who have sex with men and inject drugs (MSM-PWID) at the time of diagnosis (43% compared with 24% and 25%, respectively; P = .006). A lower proportion of surveillance referrals reported methamphetamine use compared with provider and combination referrals (16% compared with 55% and 53%, respectively; P < .001).
Table 1. .
Demographic and Clinical Characteristics of Patients Enrolled in LINCS by Referral Category at Baseline
| Total | Provider | EMR Combination List | Surveillance | P Valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | No. | % | No. | % | |
| Total | 233 | 100.0 | 167 | 100.0 | 28 | 100.0 | 38 | 100.0 | |
| Gender | .110 | ||||||||
| Male | 198 | 8.0 | 140 | 83.8 | 28 | 100.0 | 30 | 79.0 | |
| Female | 22 | 9.4 | 18 | 10.8 | 0 | 0 | 4 | 10.5 | |
| Trans women | 13 | 5.6 | 9 | 5.4 | 0 | 0 | 4 | 10.5 | |
| Trans men | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Age | .504 | ||||||||
| 13–24 y | 5 | 2.2 | 4 | 2.4 | 0 | 0 | 1 | 2.6 | |
| 25–39 y | 86 | 37.0 | 67 | 40.1 | 8 | 28.6 | 11 | 29.0 | |
| 40–49 y | 74 | 318 | 53 | 31.7 | 8 | 28.6 | 13 | 34.2 | |
| 50+ y | 68 | 29.2 | 43 | 25.8 | 12 | 42.9 | 13 | 34.2 | |
| Race | .003 | ||||||||
| White | 86 | 36.9 | 67 | 40.1 | 15 | 53.6 | 4 | 10.5 | |
| African American | 64 | 27.5 | 42 | 25.2 | 6 | 21.4 | 16 | 42.1 | |
| Latinx | 65 | 27.9 | 46 | 27.5 | 4 | 14.3 | 15 | 39.5 | |
| Other | 18 | 7.7 | 12 | 7.2 | 3 | 10.7 | 3 | 7.9 | |
| Homeless | 105 | 45.1 | 89 | 53.3 | 8 | 28.6 | 8 | 21.1 | <.001 |
| HIV risk factor at time of diagnosisa | .006 | ||||||||
| MSM | 99 | 42.5 | 64 | 38.3 | 14 | 50.0 | 21 | 55.3 | |
| PWID | 35 | 15.0 | 27 | 16.2 | 6 | 21.4 | 2 | 5.3 | |
| MSM-PWID | 87 | 37.3 | 71 | 42.5 | 7 | 25.0 | 9 | 23.7 | |
| Heterosexual | 9 | 3.9 | 3 | 1.8 | 1 | 3.6 | 5 | 13.2 | |
| Other/unknown | 3 | 1.3 | 2 | 1.2 | 0 | 0 | 1 | 2.6 | |
| SF resident at dx | 167 | 71.7 | 115 | 68.9 | 17 | 60.7 | 35 | 92.1 | .003 |
| Methamphetamine use in 12 mo before enrollment | <.001 | ||||||||
| Yes | 113 | 48.5 | 92 | 55.1 | 15 | 53.4 | 6 | 15.8 | |
| No | 56 | 24.0 | 37 | 22.2 | 4 | 14.3 | 15 | 39.5 | |
| Unknown | 64 | 27.5 | 38 | 22.8 | 9 | 32.1 | 17 | 44.7 | |
| Injection drug use in past 12 mo | .136 | ||||||||
| Yes | 71 | 30.5 | 55 | 32.9 | 10 | 35.7 | 6 | 15.8 | |
| No | 82 | 35.2 | 56 | 33.5 | 7 | 25.0 | 19 | 50.0 | |
| Unknown | 80 | 34.3 | 56 | 33.5 | 11 | 39.3 | 13 | 34.2 | |
| Length of time from HIV diagnosis | .156 | ||||||||
| <1 y | 17 | 7.3 | 16 | 9.6 | 0 | 0 | 1 | 2.6 | |
| 1–5 y | 71 | 30.5 | 54 | 32.3 | 6 | 21.4 | 11 | 29.0 | |
| 5+ y | 145 | 62.2 | 97 | 58.1 | 22 | 78.6 | 26 | 68.4 | |
| Ever suppressed | 168 | 72.1 | 122 | 73.1 | 22 | 78.6 | 24 | 63.2 | .361 |
| Percent suppressed at last VL within 12 mo before enrollment | 42 | 18.0 | 39 | 23.4 | 2 | 7.1 | 1 | 2.6 | .002 |
| CD4 cell count closest to enrollment | .629 | ||||||||
| <200 | 75 | 32.2 | 52 | 31.1 | 8 | 28.6 | 15 | 39.5 | |
| 200–349 | 52 | 22.3 | 37 | 22.2 | 6 | 21.4 | 9 | 23.7 | |
| 350–499 | 40 | 17.2 | 28 | 16.8 | 8 | 28.6 | 4 | 10.5 | |
| >500 | 66 | 28.3 | 50 | 29.9 | 6 | 21.4 | 10 | 26.3 |
The bold indicates P values that are statistically significant.
Abbreviations: EMR, electronic medical record; LINCS, Linkage, Integration, Navigation and Comprehensive Services; MSM, men who have sex with men; PWID, people who inject drugs; VL, viral load.
aFisher exact test.
Effects of Navigation on Retention and Viral Suppression
Among all PWH enrolled in LINCS, retention in care increased from 35% to 58% and viral suppression increased from 18% to 53%. The relative improvement in viral suppression among surveillance referrals was 60% (RR, 24.00; 95% CI, 3.25–177.4) compared with a relative improvement of 32% (RR, 2.36; 95% CI, 1.79–3.11) among provider referrals and 21% (RR, 4.00; 95% CI, 0.85–18.8) among combination match referrals (Table 2). Of the 140 PWH who achieved viral suppression after LINCS enrollment, 125 had enough follow-up time to evaluate durable viral suppression. Of those, 87 (70%) remained durably virally suppressed. Among PWH who had never been virally suppressed, 54% achieved viral suppression post-LINCS.
Table 2. .
Twelve Month Pre- and Postenrollment Retention and Viral Load Suppression Outcomes and Relative Risk Estimates, by Demographics and Clinical Characteristics at Baseline
| Retention | Viral Suppression | |||||
|---|---|---|---|---|---|---|
| Demographic/Behavioral Factor | % Retained Pre-LINCS | % Retained Post-LINCS | Post- vs Pre-enrollment RR (95% CI) | % Virally Suppressed 12 Months Pre- LINCS | % Virally Suppressed Within 12 Months Post- LINCS | Post- vs Pre- enrollment RR (95% CI) |
| Total (n = 233) | 35.19 | 57.51 | 1.63 (1.34–2.00) | 18.03 | 53.22 | 2.95 (2.23–3.90) |
| Age | ||||||
| ≥50 (n = 68) | 35.29 | 63.24 | 1.79 (1.29–2.49) | 19.12 | 55.88 | 2.92 (1.76–4.85) |
| 13–49 (n = 165) | 35.15 | 55.15 | 1.57 (1.22–2.02) | 17.58 | 52.15 | 2.97 (2.12–4.15) |
| Gender | ||||||
| Male (n = 198) | 34.34 | 58.59 | 1.71 (1.38–2.11) | 18.69 | 54.55 | 2.92 (2.16–3.94) |
| Female (n = 22) | 40.91 | 50 | 1.22 (0.56–2.69) | 13.64 | 54.55 | 4.00 (1.50–10.66) |
| Transgender (n = 13) | 38.46 | 53.85 | 1.40 (0.62–3.15) | 15.38 | 30.77 | 2.00 (0.50–8.00) |
| Race/ethnicity | ||||||
| White (n = 86) | 33.72 | 50 | 1.48 (1.04–2.11) | 22.09 | 47.67 | 2.16 (1.43–3.25) |
| Black (n = 64) | 37.50 | 65.63 | 1.75 (1.25–2.45) | 17.19 | 64.06 | 3.73 (2.18–6.38) |
| Latino (n = 65) | 36.92 | 60 | 1.63 (1.11–2.37) | 16.92 | 50.77 | 3.00 (1.71–5.27) |
| Other (n = 18) | 27.78 | 55.56 | 2.00 (0.80–5.02) | 5.56 | 50 | 9.00 (1.42–57.12) |
| Homeless in past 12 mo | ||||||
| Yes (n = 105) | 37.14 | 59.05 | 1.59 (1.17–2.16) | 17.14 | 49.52 | 2.89 (1.87–4.46) |
| No (n = 128) | 33.59 | 56.25 | 1.67 (1.28–2.18) | 18.75 | 56.25 | 3.00 (2.08–4.32) |
| IDU past 12 mo | ||||||
| Yes (n = 71) | 43.66 | 47.89 | 1.10 (0.76–1.58) | 22.54 | 47.89 | 2.13 (1.34–3.37) |
| No (n = 82) | 36.59 | 68.29 | 1.87 (1.37–2.54) | 15.85 | 56.1 | 3.54 (2.15–5.84) |
| Unknown (n = 80) | 26.25 | 55 | 2.09 (1.41–3.10) | 16.25 | 55 | 3.38 (2.06–5.57) |
| Methamphetamine | ||||||
| Yes (n = 113) | 35.4 | 47.79 | 1.35 (0.98–1.85) | 16.81 | 47.79 | 2.84 (1.85–4.36) |
| No (n = 56) | 42.86 | 76.79 | 1.79 (1.28–2.52) | 19.64 | 58.93 | 3.00 (1.78–5.07) |
| Unknown (n = 64) | 28.13 | 57.81 | 2.06 (1.37–3.09) | 18.75 | 57.81 | 3.08 (1.84–5.18) |
| Ever suppressed | ||||||
| Yes (n = 168) | 43.45 | 57.74 | 1.33 (1.08–1.63) | 25 | 52.98 | 2.12 (1.62–2.78) |
| No (n = 65) | 13.85 | 56.92 | 4.11 (2.12–7.97) | 0 | 53.85 | NA |
| Referral source | ||||||
| Provider (n = 167) | 40.12 | 58.08 | 1.45 (1.16–1.81) | 23.35 | 55.09 | 2.36 (1.79–3.11) |
| Surveillance (n = 38) | 31.58 | 71.05 | 2.25 (1.37–3.71) | 2.63 | 63.16 | 24.00 (3.25–177.40) |
| Combination (n = 28) | 10.71 | 35.71 | 3.33 (1.02–10.92) | 7.14 | 28.57 | 4.00 (0.85–18.84) |
Abbreviations: IDU, injection drug use; LINCS, Linkage, Integration, Navigation and Comprehensive Services; RR, relative risk.
Viral suppression increased by 47% among blacks, 34% among Latinx, 31% among methamphetamine users, and 27% among people experiencing homelessness. Notably, there were no differences in relative improvements by race, methamphetamine use, or housing status (Table 2). Transgender PWH did not have significant improvements in retention and viral suppression after enrollment in LINCS, although the overall number enrolled was small.
DISCUSSION
To our knowledge, this is the first report from an HIV navigation program that compares retention and viral suppression outcomes between 3 D2C referral strategies implemented contemporaneously. Across all 3 referral strategies, over a quarter (28%) of enrolled PWH had no evidence of prior viral suppression, and 54% of these patients achieved viral suppression within 12 months after the intervention. The relative improvements in viral suppression were significant across many key covariates. These data reinforce that public health re-linkage efforts can address health disparities, as we found that blacks, Latinx individuals, methamphetamine users, and people experiencing homelessness who were enrolled in LINCS experienced improvements in viral suppression.
Similar to what has been previously reported, we found that referrals from surveillance-generated lists were both the least accurate (ie, most likely to include persons already in care) and least efficient [13]. In order to enroll 1 NIC PWH in LINCS, 11 surveillance referrals were investigated, compared with 3 provider or 5 combination match referrals. However, the improvement in the proportion of individuals who were virally suppressed was highest among those identified through surveillance (+60.5%) compared with those identified by providers (+31.7%) or combination lists (+21.4%). This is partly explained by the fact that PWH who were identified through surveillance were much less likely to have been virally suppressed in the year before LINCS enrollment (2.6% vs 23.6% vs 7.1%.) Interestingly, although the vast majority of surveillance referrals were not virally suppressed in the year before LINCS, they also were the least likely to report methamphetamine use (15.8%) or homelessness (21.1%). The low prevalence of these barriers to care among PWH enrolled from surveillance likely contributes to the high level of retention (71%) and viral suppression (63%) in the 12 months after LINCS enrollment [23].
We had hypothesized that the combination match would yield the highest efficiency because, by matching an EMR-derived registry to surveillance, we could prioritize the patients most likely to be locatable and eligible for re-linkage. However, 48% of combination match referrals were living out of jurisdiction or could not be located, compared with 42% of surveillance and 26% of provider referrals. In New York State, separate programs using different referral strategies similarly found lower re-linkage outcomes among referrals identified by partnering health centers as compared with surveillance referrals [17]. Our combination list was derived from the clinical EMR of public health clinics that made frequent provider referrals to LINCS, which may have resulted in over-representation of individuals who were not previously referred to LINCS because they require more long-term interventions or have decreased access to services because they reside outside of San Francisco.
This is a program evaluation, and there are several limitations to the generalizability of our data. Importantly, there was no control group, and we cannot compare our results to an expected change in viral suppression that would have occurred without our efforts. To date, controlled evaluations of the effect of navigation on retention and suppression outcomes have had null results [12, 24, 25]. There are data to support that many PWH “churn” in and out of care, and the cyclical return to care could be contributing to the change we observed [26]. Despite the potential impact of churn on improvements in viral suppression, we demonstrated an improvement in retention in care post-LINCS across all referral strategies, supporting that re-linked PWH attended subsequent care visits in the same year. The impact of this short-term intervention on long-term outcomes is also supported by the finding that 70% of PWH who achieved viral suppression remained durably virally suppressed. We do not have complete data on barriers to care, particularly substance use (28% missing data among enrollees) and mental health (not routinely collected), which are clearly associated with HIV care engagement [23]. Finally, we did not measure time spent or cost involved to generate lists and investigate and re-link PWH, which is needed to prioritize and implement D2C programs [27].
A major strength of our study is that we utilized multiple referral strategies contemporaneously within the same program and evaluated care continuum outcomes following enrollment in LINCS using surveillance data. To date, most HIV navigation program data have not been systematically analyzed, and there is marked heterogeneity in outcome reporting [22]. Prior evaluations of navigation efforts using public health surveillance data included NIC PWH with similar or higher pre-enrollment viral suppression than our cohort (17% in Seattle [28], 32% in New York City [29], 43% in Massachusetts [18], and 51% in Los Angeles County [30]). A unique aspect of our program is that some LINCS navigators were placed within public HIV clinics and built trust with clinical teams while also conducting field outreach and utilizing multiple data systems to locate patients. By strengthening the collaboration between the health department navigators and HIV care teams over time, providers better understood the capability of LINCS and more readily initiated referrals of loosely engaged and NIC PWH to navigation [31].
HIV disparities are amplifying in San Francisco, and creative approaches to improve viral suppression are needed [2]. While surveillance data alone are not an efficient way to identify candidates for re-linkage services, the viral suppression outcomes from this referral source are impressive and reaffirm that surveillance data can identify individuals who benefit from navigation. In our program, the abundance of provider referrals reflects the importance of systematic panel management to detect disengagement from care [31]. For re-linkage efforts to lead to long-term engagement, health systems must have robust panel management, including appointment reminders, missed visit follow-up, and case management services [32]. Innovative differentiated care models are also needed in order to re-link PWH to the appropriate level of care that will help ensure durable viral suppression. The Max Clinic in Seattle & King County and the POP-UP clinic at Ward 86/Zuckerberg General Hospital in San Francisco offer drop-in or open-access HIV primary care that may meet the needs of PWH who cannot attend scheduled appointments or do not virally suppress after re-linkage efforts [28, 31, 33].
In the era of Ending the HIV Epidemic, public health departments are charged with addressing disparities in viral suppression and expanding data-driven approaches to identify PWH who are NIC and provide support to re-link and engage them in ongoing HIV care [1]. Currently, the LINCS program prioritizes provider referrals due to ongoing high demand for navigation by motivated providers seeking to re-engage PWH, in addition to the known efficiencies over other D2C referral strategies. By strengthening relationships with clinical and case management providers and leveraging multiple data sources (including clinical record systems), re-linkage services can be a timely, proactive intervention, rather than a safety net after patients are fully disengaged from the health care system. Outcomes from first-generation D2C efforts shed light on the opportunities and challenges of utilizing surveillance data to improve HIV outcomes. In order to capitalize on opportunities and fully realize the goal of no new infections, it will take significant and sustained efforts by care providers, health care systems, and health departments to improve continuity of care and ensure lifelong viral suppression.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. We would like to acknowledge financial support from the Centers for Disease Control and Prevention grant “San Francisco PrEP and Data to Care Demonstration Project” (NU62PS005027) and from the M.A.C AIDS grant “Getting to Zero: Advancing Retention and Reengagement in HIV Care Demonstration Project” (N-T-YY-21698).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 2. Lesko CR, Sampson LA, Miller WC, et al. Measuring the HIV care continuum using public health surveillance data in the United States. J Acquir Immune Defic Syndr 2015; 70:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Udeagu C, Huang J, Eason L, Pickett L. Health department-HIV clinic integration of data and human resources to re-engage out of care HIV-positive persons into clinical care in a New York City locale. AIDS Care 2019; 31:1420–6. [DOI] [PubMed] [Google Scholar]
- 4. Li Z, Purcell DW, Sansom SL, et al. Vital signs: HIV transmission along the continuum of care—United States, 2016. MMWR Morb Mortal Wkly Rep 2019; 68:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS 2007; 21(Suppl 1):S49–58. [DOI] [PubMed] [Google Scholar]
- 6. Using HIV surveillance data to support the HIV care continuum. 2015. Available at: https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/HIV-Epidemiology-Annual-Report-2018.pdf. Accessed 30 August 2020.
- 7. Sweeney P, DiNenno EA, Flores SA, et al. HIV data to care—using public health data to improve HIV care and prevention. J Acquir Immune Defic Syndr 2019; 82(Suppl 1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CDC Effective Interventions. Data to care: essential elements 2019. Available at: https://effectiveinterventions.cdc.gov/data-to-care/group-1/data-to-care/data-to-care-essential-elements. Accessed 26 December 2019.
- 9. Sitapati AM, Limneos J, Bonet-Vázquez M, et al. Retention: building a patient-centered medical home in HIV primary care through PUFF (patients unable to follow-up found). J Health Care Poor Underserved 2012; 23:81–95. [DOI] [PubMed] [Google Scholar]
- 10. Bove JM, Golden MR, Dhanireddy S, et al. Outcomes of a clinic-based surveillance-informed intervention to relink patients to HIV care. J Acquir Immune Defic Syndr 2015; 70:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Udeagu CC, Webster TR, Bocour A, et al. Lost or just not following up: public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care. AIDS 2013; 27:2271–9. [DOI] [PubMed] [Google Scholar]
- 12. Dombrowski JC, Hughes JP, Buskin SE, et al. A cluster randomized evaluation of a health department data to care intervention designed to increase engagement in HIV care and antiretroviral use. Sex Transm Dis 2018; 45:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tesoriero JM, Johnson BL, Hart-Malloy R, et al. Improving retention in HIV care through New York’s expanded partner services data-to-care pilot. J Public Health Manag Pract 2017; 23:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchacz K, Chen MJ, Parisi MK, et al. Using HIV surveillance registry data to re-link persons to care: the RSVP Project in San Francisco. PLoS One 2015; 10:e0118923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertolli J, Garland PM, Valverde EE, et al. ; Never in Care Pilot Project Team Missed connections: HIV-infected people never in care. Public Health Rep 2013; 128:117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hague JC, John B, Goldman L, et al. Using HIV surveillance laboratory data to identify out-of-care patients. AIDS Behav 2019; 23:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hart-Malloy R, Brown S, Bogucki K, Tesoriero J. Implementing data-to-care initiatives for HIV in New York state: assessing the value of community health centers identifying persons out of care for health department follow-up. AIDS Care 2018; 30:391–6. [DOI] [PubMed] [Google Scholar]
- 18. Kunzweiler C, Kishore N, John B, et al. Using HIV surveillance and clinic data to optimize data to care efforts in community health centers in Massachusetts: the Massachusetts Partnerships for Care Project. J Acquir Immune Defic Syndr 2019; 82(Suppl 1):33–41. [DOI] [PubMed] [Google Scholar]
- 19. Arey AL, Cassidy-Stewart H, Kurowski PL, et al. Evaluating HIV surveillance completeness along the continuum of care: supplementing surveillance with health center data to increase HIV data to care efficiency. J Acquir Immune Defic Syndr 2019; 82(Suppl 1):26–32. [DOI] [PubMed] [Google Scholar]
- 20. Dombrowski JC, Bove J, Roscoe JC, et al. ; Northwest Health DepartmentCenters for AIDS Research (CFAR) Consortium “Out of care” HIV case investigations: a collaborative analysis across 6 states in the Northwest US. J Acquir Immune Defic Syndr 2017; 74(Suppl 2):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hart-Malloy R, Shrestha T, Pezzulo MC, et al. Data to care opportunities: an evaluation of persons living with HIV reported to be “current to care” without current HIV-related labs. J Acquir Immune Defic Syndr 2019; 82(Suppl 1):20–5. [DOI] [PubMed] [Google Scholar]
- 22. Mizuno Y, Higa DH, Leighton CA, et al. Is HIV patient navigation associated with HIV care continuum outcomes? AIDS 2018; 32:2557–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bulsara SM, Wainberg ML, Newton-John TRO. Predictors of adult retention in HIV care: a systematic review. AIDS Behav 2018; 22:752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cabral HJ, Davis-Plourde K, Sarango M, et al. Peer support and the HIV continuum of care: results from a multi-site randomized clinical trial in three urban clinics in the United States. AIDS Behav 2018; 22:2627–39. [DOI] [PubMed] [Google Scholar]
- 25. Metsch LR, Feaster DJ, Gooden L, et al. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. JAMA 2016; 316:156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nosyk B, Lourenço L, Min JE, et al. ; STOP HIVAIDS Study Group Characterizing retention in HAART as a recurrent event process: insights into ‘cascade churn.’ AIDS 2015; 29:1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neblett Fanfair R, Shrestha RK, Randall L, et al. Implementing data to care—what are the costs for the health department? J Acquir Immune Defic Syndr 2019; 82(Suppl 1):57–61. [DOI] [PubMed] [Google Scholar]
- 28. Chang EJ, Fleming M, Nunez A, Dombrowski JC. Predictors of successful HIV care re-engagement among persons poorly engaged in HIV care. AIDS Behav 2019; 23:2490–7. [DOI] [PubMed] [Google Scholar]
- 29. Irvine MK, Chamberlin SA, Robbins RS, et al. Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clin Infect Dis 2015; 60:298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wohl AR, Dierst-Davies R, Victoroff A, et al. Implementation and operational research: the navigation program: an intervention to reengage lost patients at 7 HIV clinics in Los Angeles County, 2012-2014. J Acquir Immune Defic Syndr 2016; 71:e44–50. [DOI] [PubMed] [Google Scholar]
- 31. Johnson MO, Neilands TB, Koester KA, et al. Detecting disengagement from HIV care before it is too late: development and preliminary validation of a novel index of engagement in HIV care. J Acquir Immune Defic Syndr 2019; 81:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pence BW, Bengtson AM, Boswell S, et al. Who will show? Predicting missed visits among patients in routine HIV primary care in the United States. AIDS Behav 2019; 23:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dombrowski JC, Galagan SR, Ramchandani M, et al. HIV care for patients with complex needs: a controlled evaluation of a walk-in, incentivized care model. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.