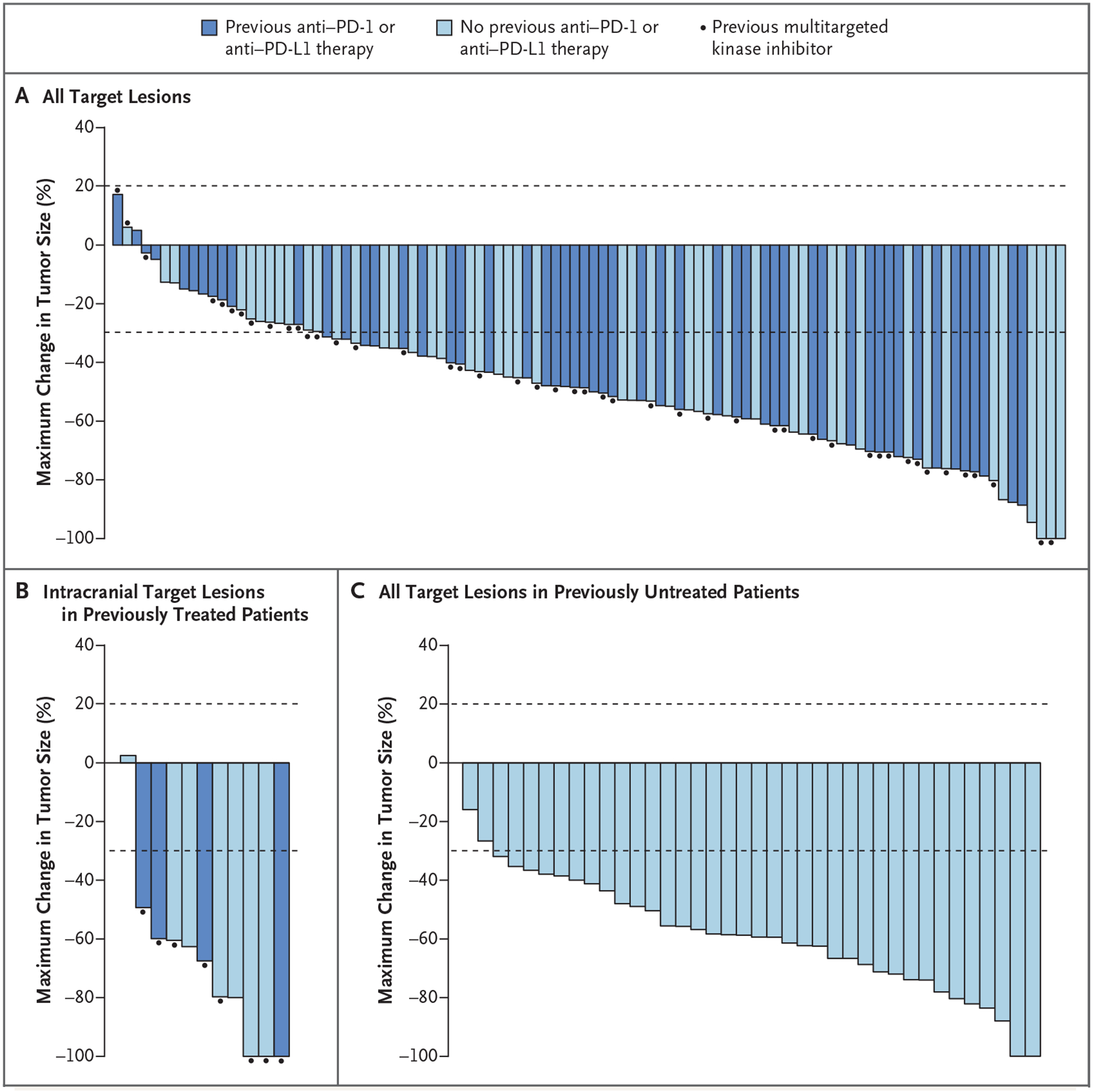
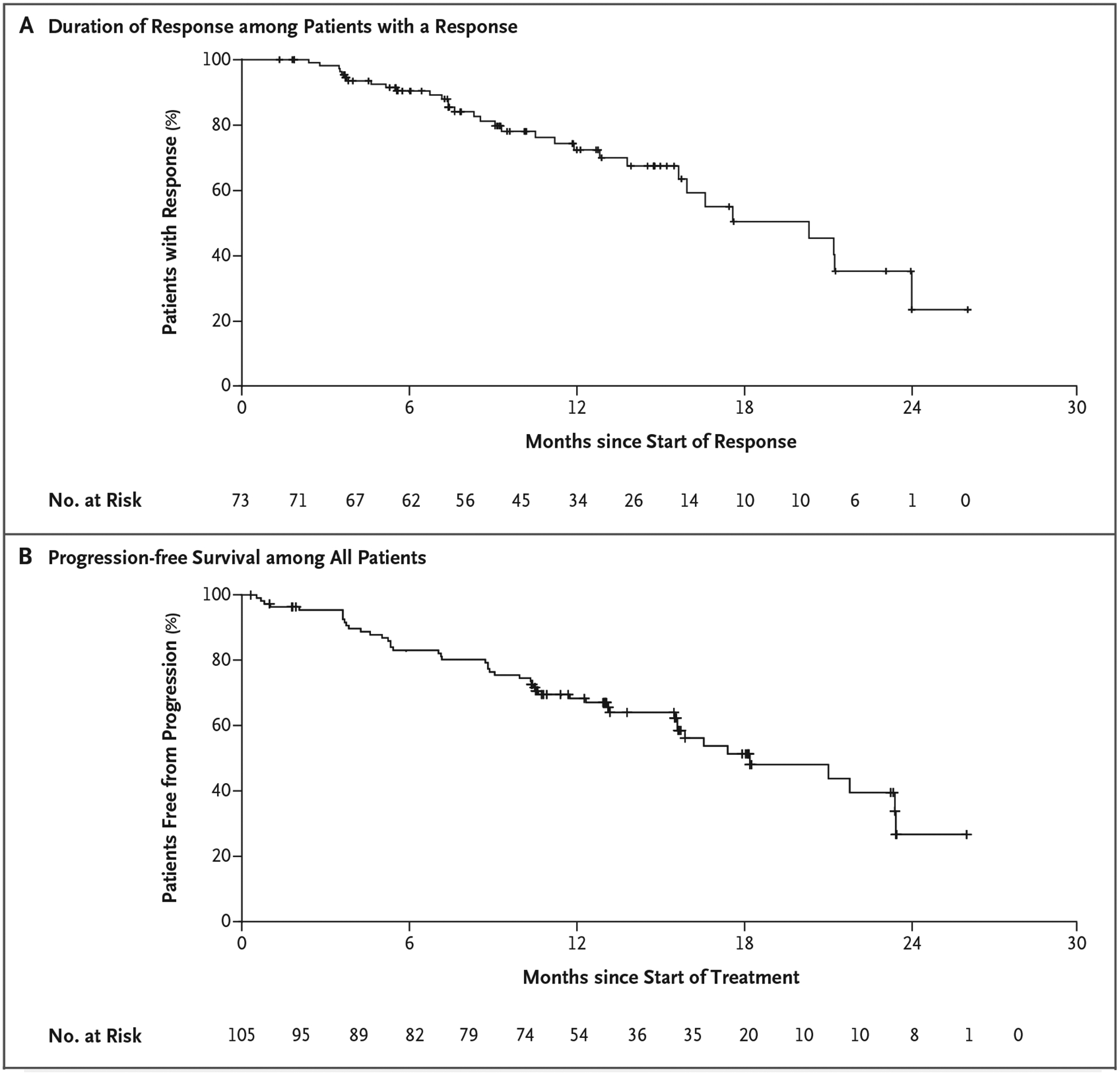
Alexander Drilon
Alexander Drilon, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,#,
Geoffrey R Oxnard
Geoffrey R Oxnard, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,#,
Daniel SW Tan
Daniel SW Tan, M.B., B.S., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,#,
Herbert HF Loong
Herbert HF Loong, M.B., B.S.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Melissa Johnson
Melissa Johnson, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Justin Gainor
Justin Gainor, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Caroline E McCoach
Caroline E McCoach, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Oliver Gautschi
Oliver Gautschi, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Benjamin Besse
Benjamin Besse, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Byoung C Cho
Byoung C Cho, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Nir Peled
Nir Peled, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Jared Weiss
Jared Weiss, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Yu-Jung Kim
Yu-Jung Kim, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Yuichiro Ohe
Yuichiro Ohe, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Makoto Nishio
Makoto Nishio, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Keunchil Park
Keunchil Park, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Jyoti Patel
Jyoti Patel, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Takashi Seto
Takashi Seto, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Tomohiro Sakamoto
Tomohiro Sakamoto, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Ezra Rosen
Ezra Rosen, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Manisha H Shah
Manisha H Shah, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Fabrice Barlesi
Fabrice Barlesi, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Philippe A Cassier
Philippe A Cassier, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Lyudmila Bazhenova
Lyudmila Bazhenova, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Filippo De Braud
Filippo De Braud, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Elena Garralda
Elena Garralda, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Vamsidhar Velcheti
Vamsidhar Velcheti, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Miyako Satouchi
Miyako Satouchi, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Kadoaki Ohashi
Kadoaki Ohashi, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Nathan A Pennell
Nathan A Pennell, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Karen L Reckamp
Karen L Reckamp, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Grace K Dy
Grace K Dy, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Jürgen Wolf
Jürgen Wolf, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Benjamin Solomon
Benjamin Solomon, M.B., B.S., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Gerald Falchook
Gerald Falchook, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Kevin Ebata
Kevin Ebata, Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Michele Nguyen
Michele Nguyen, B.S.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Binoj Nair
Binoj Nair, Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Edward Y Zhu
Edward Y Zhu, Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Luxi Yang
Luxi Yang, M.P.H.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Xin Huang
Xin Huang, Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Elizabeth Olek
Elizabeth Olek, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
S Michael Rothenberg
S Michael Rothenberg, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Koichi Goto
Koichi Goto, M.D., Ph.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1,
Vivek Subbiah
Vivek Subbiah, M.D.
1Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (A.D., E.R.) and New York University Langone Medical Center (V.V.), New York, and Roswell Park Comprehensive Cancer Center, Buffalo (G.K.D.) — all in New York; Dana–Farber Cancer Institute (G.R.O.) and Massachusetts General Hospital (J.G.), Boston; National Cancer Centre Singapore, Singapore (D.S.W.T.); the Chinese University of Hong Kong, Hong Kong, China (H.H.F.L.); Sarah Cannon Research Institute, Nashville (M.J.); University of California, San Francisco–Helen Diller Family Comprehensive Cancer Center, San Francisco (C.E.M.), University of California, San Diego, Moores Cancer Center, La Jolla (L.B.), and City of Hope Comprehensive Cancer Center, Duarte (K.L.R.) — all in California; University of Bern, Bern, and Cantonal Hospital of Lucerne, Lucerne — both in Switzerland (O.G.); Institut Gustave Roussy, Villejuif (B.B.), Hospital La Timone, Marseille (F.B.), and Centre Léon Bérard, Lyon (P.A.C.) — all in France; Severance Hospital, Yonsei University Health System (B.C.C.), and Samsung Medical Center, Sungkyunkwan University School of Medicine (K.P.), Seoul, and Seoul National University Bundang Hospital, Seongnam (Y.J.K.) — all in South Korea; Soroka University Medical Center, Beer-Sheva, Israel (N.P.); University of North Carolina–Chapel Hill, Chapel Hill (J. Weiss); National Cancer Center Hospital (Y.O.) and Cancer Institute Hospital of the Japanese Foundation for Cancer Research (M. Nishio), Tokyo, National Hospital Organization Kyushu Cancer Center, Fukuoka (T. Seto), Tottori University Hospital, Tottori (T. Sakamoto), Hyogo Cancer Center, Akashi (M.S.), Okayama University Hospital, Okayama (K.O.), and National Cancer Center Hospital East, Chiba (K.G.) — all in Japan; University of Chicago, Chicago (J.P.); the Ohio State University Comprehensive Cancer Center, Columbus (M.H.S.), and Cleveland Clinic, Cleveland (N.A.P.); Istituto Nazionale Tumori–National Cancer Institute, Università degli Studi di Milano, Milan (F.D.B.); Vall d’Hebron Institute of Oncology, Hospital Universitario Vall d’Hebron, Barcelona (E.G.); Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J. Wolf); Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Sarah Cannon Research Institute at HealthONE, Denver (G.F.); Loxo Oncology, Stamford, CT (K.E., M. Nguyen, B.N., E.Y.Z., L.Y., X.H., E.O., S.M.R.); and the University of Texas M.D. Anderson Cancer Center, Houston (V.S.).
1