Figure 4. Identification of ATX-O-methyl oxime (M30).
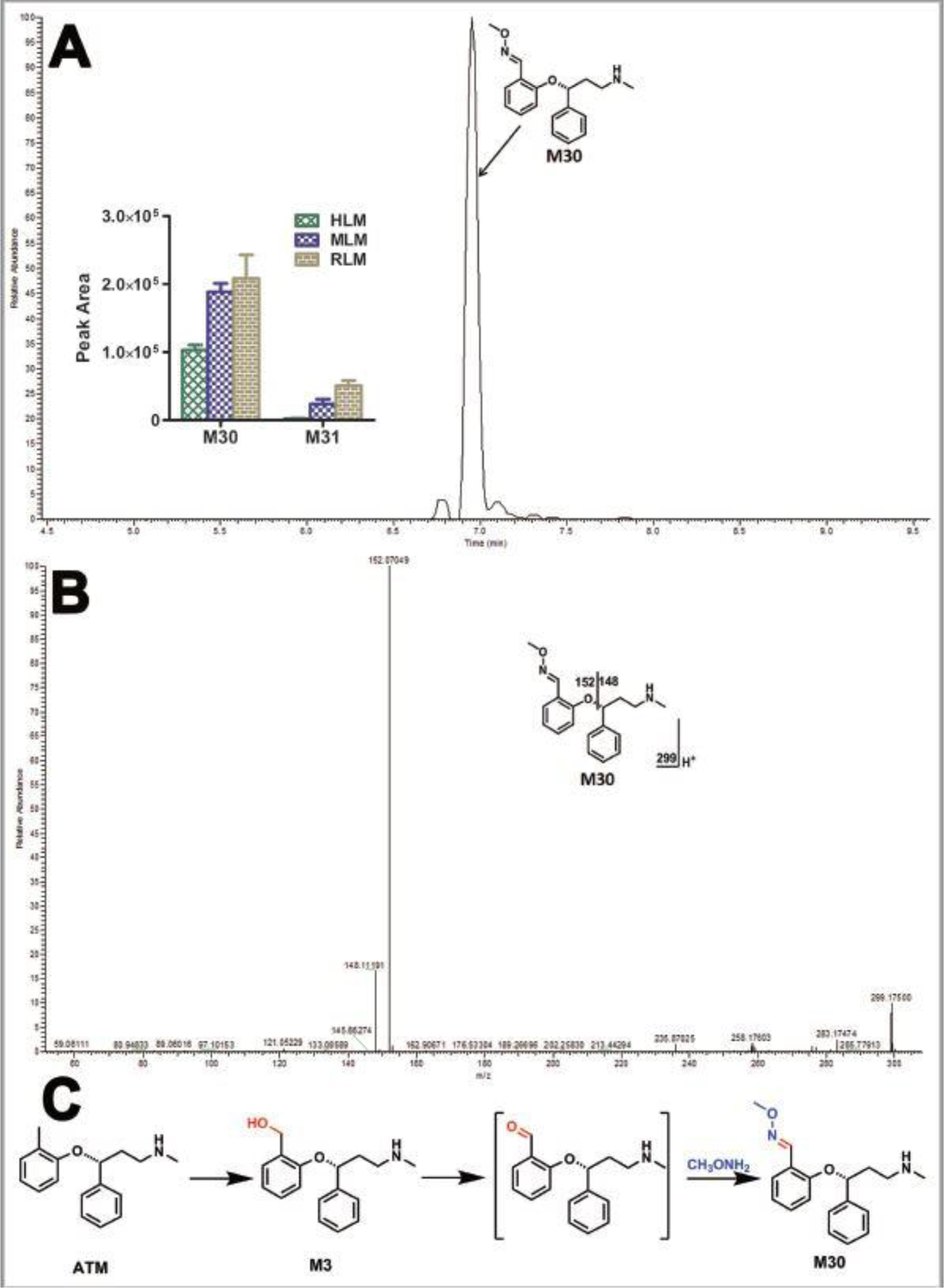
Incubations were conducted in 1X phosphate-buffered saline (1X PBS, pH 7.4), containing 25 µM ATX, 0.2 mg HLM, 2.5 mM methxoyamine, and NADPH (final concentration 1.0 mM) in a final volume of 200 µl. All the samples were analyzed using UHPLC-Q Exactive MS. Structural elucidations were performed based on accurate mass (mass errors less than 5 ppm) and MS/MS fragmentation. MS/MS was performed with collision energy ramping from 10–30 V. The major fragmental ions are interpreted in the insets. (A) Chromatograms of M30 and the relative abundance of M30 and M31 in LMs. (B) MS/MS of M30. (C) Proposed mechanism of the aldehyde formation and trapping strategy. ATX-O-methyl oxime (M30) was produced in three steps: 1) monohydroxylation of ATX to form M3; 2) further oxidation to generate the aldehyde; 3) reacted with methoxyamine to form M30.
