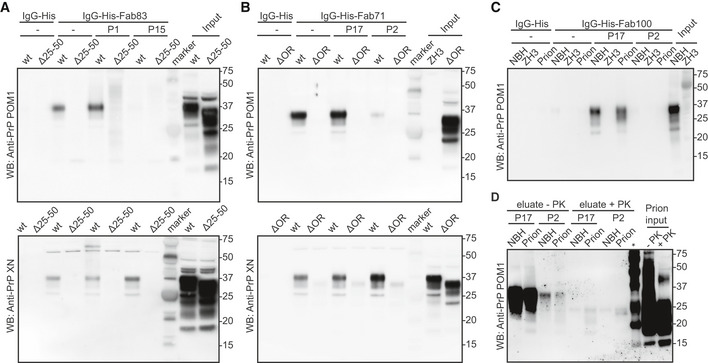
Figure EV4. Validation of the reactivity of the anti‐PrP Fabs by immunoprecipitation.
-
AFab83 coupled beads immunoprecipitated wtPrPC from NBH, but not from BH of PrPΔ25–50 mice lacking the respective epitope. WtPrPC specifically eluted by competition with the Fab83 epitope‐targeting peptide P1 (residues 23–34), but not with the unrelated peptide P15 (top panel). Elution with a SDS buffer was used as nonspecific control (lower panel). Control: IgG‐His. Molecular sizes are presented in kDa.
-
BSame as (A), but for Fab71 coupled beads which immunoprecipitated wtPrPC from NBH, but not from BHs of PrPΔOR mice. WtPrPC specifically eluted with the epitope‐targeting peptide P17 (residues 55–66), but not with the unrelated peptide P2 (top panel).
-
CIgG‐His-Fab100 coupled beads efficiently immunoprecipitated wtPrPC from non‐infectious brain homogenate (NBH) and total wtPrP from brains of prion‐infected mice (prion), but not from brains of Prnp ZH3/ZH3 (ZH3) mice. WtPrP was eluted by competition with the epitope‐targeting peptide P17, but not with the unrelated peptide P2. Eluates were visualized by Western blotting. Control: IgG‐His. Molecular sizes are presented in kDa.
-
DSame as (C) for NBH and prion to confirm the specific immunoprecipitation and detection of PrPSc with the peptide P17 from brains of prion‐infected mice. +PK: digested with PK; −PK: non‐digested with PK; *: molecular weight markers.
