Abstract
Electrogastrography (EGG) is the noninvasive electrophysiological technique used to record gastric electrical activity by means of cutaneous electrodes placed on the abdomen. EGG has been so far mostly used in clinical studies in gastroenterology, but it represents an attractive method to study brain‐viscera interactions in psychophysiology. Compared to the literature on electrocardiography for instance, where practical recommendations and normative data are abundant, the literature on EGG in humans remains scarce. The aim of this article is threefold. First, we review the existing literature on the physiological basis of the EGG, pathways of brain‐stomach interactions, and experimental findings in the cognitive neuroscience and psychophysiology literature. We then describe practical issues faced when recording the EGG in young healthy participants, from data acquisition to data analysis, and propose a semi‐automated analysis pipeline together with associated MATLAB code. The analysis pipeline aims at identifying a regular rhythm that can be safely attributed to the stomach, through multiple steps. Finally, we apply these recording and analysis procedures in a large sample (N = 117) of healthy young adult male and female participants in a moderate (<5 hr) to prolonged (>10 hr) fasting state to establish the normative distribution of several EGG parameters. Our results are overall congruent with the clinical gastroenterology literature, but suggest using an electrode coverage extending to lower abdominal locations than current clinical guidelines. Our results indicate a marginal difference in EGG peak frequency between male and female participants, and that the gastric rhythm becomes more irregular after prolonged fasting.
Keywords: electrogastrography, gastric rhythm, normative data, power and phase analysis, processing pipeline
Short abstract
The electrogastrogram, which reflects the electrical activity of the stomach, is a measure of interest in psychophysiology. We provide here practical guidelines for recording, processing and analyzing this signal. The procedures are applied in a large dataset (n = 117) to provide normative values in young, healthy adults and analyze inter‐subject variability.
1. AN INTRODUCTION TO ELECTROGASTROGRAPHY
1.1. The electrogastrogram and its usage
“Electrogastrography” refers to the monitoring technique of gastric myoelectrical activity from cutaneous electrodes placed on the abdomen (Figure 1a), which generates the electrogastrogram (EGG). The EGG has so far mostly been used for clinical purposes in gastroenterology (Koch & Stern, 2004; Parkman, Hasler, Barnett, & Eaker, 2003; Riezzo, Russo, & Indrio, 2013; Yin & Chen, 2013), but represents an interesting tool in psychophysiology (Stern, Koch, Levine, & Muth, 2007; Stern, Koch, Stewart, & Vasey, 1987). The EGG reflects the combination of the slow electrical gastric rhythm, constantly generated in the stomach wall, and of the more transient smooth muscle activity generating gastric peristaltic contractions. The main function of the stomach is to mix and grind food during digestion. The gastric rhythm sets the frequency of smooth muscle contractions and controls their propagation. The gastric rhythm is constantly generated in the stomach wall, even in the absence of muscular contraction (Bozler, 1945), or when the stomach is completely disconnected from the central nervous system (Suzuki, Prosser, & Dahms, 1986). Its normal frequency in healthy humans is around 0.05 Hz or three cycles per minute, that is, one cycle every 20 s. EGG frequency differs in other species (Mice: 2–5 cpm [Hou, Yin, Liu, Pasricha, & Chen, 2005]; Pigs: ~3.3 cpm [Květina et al., 2010; Varayil et al., 2009]; dogs: 4–6.5 cpm [Andreis et al., 2008; Mintchev, Otto, & Bowes, 1997]; macaque monkeys: ~3.6 cpm [Linsong, Huailin, Xitai, Xiaojin, & Pingan, 1989]).
Figure 1.
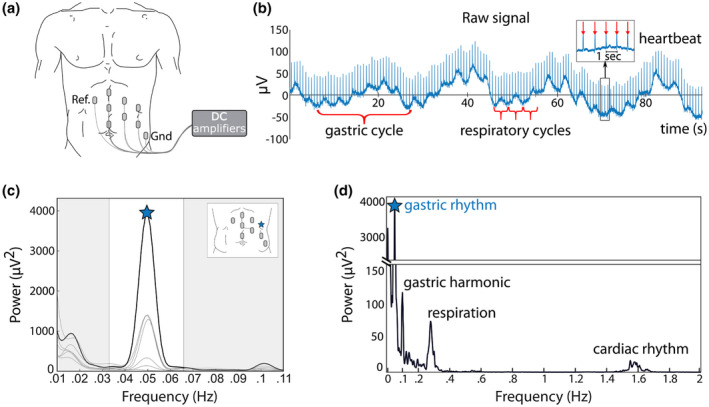
(a) Recording setup. Cutaneous electrodes are placed on the left abdomen of the participant in a grid‐like arrangement and connected to DC amplifiers. Ref. and Gnd correspond to Reference and Ground, respectively. (b) Example of raw data in one participant, where the gastric rhythm is visible as cycles of ~20 s length. Respiratory cycles are much faster (typically 3 to 5 s length). Heartbeats appear as transients every ~0.8 s (inset). EGG amplitude in this participant is close the median value observed in 100 participants. (c) Power spectrum at each of the seven recording electrodes. Peak frequency is indicated by a star on the channel with the largest power (black line). The white area corresponds to the normal frequency range of the EGG, also known as normogastria (2 to 4 cpm or 0.033 to 0.066 Hz). Inset: Electrode layout with the location of the electrode displaying the largest spectral power marked with a blue star. (d) Spectral density over a wider frequency range at the selected channel, revealing the spectral signatures of the respiratory (~0.3 Hz) and cardiac rhythms (~1.5 Hz)
The definition of normogastria, or normal frequency range of the gastric rhythm in humans, varies depending on authors (for review, Chang, 2005; Parkman et al., 2003). Along with a number of studies (e.g., Chen & McCallum, 1992; Chen, Zou, Lin, Ouyang, & Liang, 1999; Lin, 1999; Parkman, Harris, Miller, & Fisher, 1996; Parkman et al., 2003; Pfaffenbach, Adamek, Kuhn, & Wegener, 1995; Riezzo, Chiloiro, & Guerra, 1998) and guidelines (e.g., Yin & Chen, 2013), we adopted the 2–4 cpm cycles per minute (cpm) range. A narrower range has also been advocated (e.g., 2.5 to 3.6 cpm in Koch & Stern, 2004), and a number of studies used ranges closer to this definition (e.g., Abell & Malagelada, 1988; Gianaros, Quigley, & Mordkoff, 2001; Homma et al., 1999; Koch, Bingaman, Tan, & Stern, 1998; Koch, Hong, & Xu, 2000; Meissner, Muth, & Herbert, 2011; Muth, Koch, Stern, & Thayer, 1999; Stern, Vasey, Senqi, & Koch, 1991; Vianna, Weinstock, Elliott, Summers, & Tranel, 2006).
An example of a raw signal obtained from cutaneous abdominal electrodes is shown in Figure 1b. In this good quality recording, the gastric rhythm is visible to the naked eye. In the same raw data, the faster rhythms of respiration (typically around 0.2–0.4 Hz) (Kaiho, Shimoyama, Nakajima, & Ochiai, 2000) and heartbeats (1–1.7 Hz) can be observed, superimposed on the gastric rhythm (Abell & Malagelada, 1988; Stern et al., 1987). The spectral analysis of the EGG reveals a sharp peak around 0.05 Hz (Figure 1c). The EGG spectral signature is markedly distinct from those of respiration and heart rate, that peak at much higher frequencies (Figure 1d). Note that a harmonic of the gastric rhythm can sometimes be observed (Figure 1d) when the EGG departs from a perfect sine wave (Verhagen, Van Schelven, Samsom, & Smout, 1999).
Historically, the EGG was independently discovered by Alvarez, 1922; Davis, Garafolo, & Kveim, 1959; Tumpeer & Blitsten, 1926. While this recording technique received little attention for decades, computerized analysis rekindled interest in the 1990s in the field of gastroenterology (Koch & Stern, 2004). EGG recording and analysis has been first and mostly performed in the clinical domain, where it represents an appealing method since it is noninvasive, cheap, and relatively easy to install and acquire. In gastroenterology, the EGG is typically acquired before and after a meal, called the pre‐ and postprandial period, respectively. The EGG amplitude normally increases in the postprandial period in healthy participants, while EGG frequency remains relatively unaffected (for review see Koch & Stern, 2004; Riezzo et al., 2013; Stern et al., 1987). Gastroenterologists have been interested in characterizing EGG abnormalities in patients by describing changes in power and frequency. For instance, postprandial increases in EGG amplitude are altered in gastric motility disorders (Cucchiara et al., 1997; Parkman & Orr, 2007) and Parkinson's disease (Kaneoke et al., 1995). Other studies analyzed changes in EGG frequency. The gastric rhythm tends to get faster (tachygastria) in patients with nausea (Geldof et al., 1989), depression (Ruhland et al., 2008), and schizophrenia (Peupelmann et al., 2009). Note that different approaches have been used to characterize departure from normogastria, either by analyzing the percentage distribution of EGG power in different frequency bands or by analyzing the shifts of EGG peak frequency over time (Stern et al., 2007). Here, we will focus on preprandial EGG recordings in healthy participants.
1.2. The EGG in psychophysiology
The potential relevance of visceral signals for understanding brain and behavior has long been underlined for emotions (Cannon, 1927; Damasio, 1996; James, 1890; Lange, 1885), but also in a relationship with self and consciousness (Azzalini, Rebollo, & Tallon‐Baudry, 2019; Christoff, Cosmelli, Legrand, & Thompson, 2011; Craig, 2002; Critchley & Harrison, 2013; Thompson & Varela, 2001), as well as in physical and mental health (Khalsa et al., 2018; Quadt, Critchley, & Garfinkel, 2018). Note that brain‐viscera interplay ranges from the implicit nonconscious signaling of bodily afferents to the brain and/or automatic descending modulation of stomach activity by the brain to explicit or consciously accessible visceral perception (Azzalini et al., 2019; Quadt et al., 2018).
Despite the potential relevance of brain‐viscera relationships, empirical studies investigating the electrical activity of the gastrointestinal system remain scarce and provided mixed results. Initial studies on shock/noise avoidance reported mixed results on EGG amplitude (Davis & Berry, 1963; Fedor & Russell, 1965; Stern, 1966, 1983; White, 1964). Different physical and psychological stressors were found to increase spectral power in the tachygastric range (Gianaros et al., 2001; Muth et al., 1999; for conflicting results see Riezzo, Porcelli, Guerra, & Giorgio, 1996; Stern et al., 1991), while the effects on amplitude have been mixed (Riezzo et al., 1996; Stern et al., 1991). In line with the results of stressors, videos evoking disgust were found to evoke tachygastria (Harrison, Gray, Gianaros, & Critchley, 2010) but this result was not replicated (Meissner et al., 2011). Emotions induced by movie clips (or music) most often do not alter the EGG frequency (Baldaro et al., 1996, 2001; Baldaro, Battacchi, Trombini, Palomba, & Stegagno, 1990; Chen, Xu, Wang, & Chen, 2005; Chen et al., 2008; Lin et al., 2007), while results on amplitude are inconsistent (Baldaro et al., 1996, 2001; Chen et al., 2005, 2008; Lin et al., 2007; Vianna et al., 2006). Several studies found increased mean EGG power during the performance of tasks of mental arithmetic (Davis, Berry, & Paden, 1969; Holzl, Schroder, & Kiefer, 1979; Riezzo et al., 1996; for conflicting null finding see Walker & Sandman, 1977), but other studies on mental arithmetic and puzzle‐solving report fewer episodes of large amplitude gastric activity (Ercolani et al., 1982; Ercolani, Baldaro, & Trombini, 1989; Martin, Nicolov, Ormieres, Beloncle, & Murat, 1982). Different factors, like the type of task, or fed versus fasted state of participants, number of participants as well as interindividual variability (Riezzo et al., 1996) might account for discrepancies between different studies. The absence of standardized procedures in psychophysiology for recording and analyzing the EGG might play an additional role (but see e.g., Koch et al., 2000 for reproducibility in the water load test).
In addition to a potential modulation of EGG parameters by descending cognitive influences, ascending influences arising from the stomach were recently shown to influence brain dynamics at rest, with a modulation of the amplitude of the alpha rhythm by the phase of the EGG (Richter, Babo‐Rebelo, Schwartz, & Tallon‐Baudry, 2017) in the parieto‐occipital region. fMRI data reveal that the brain at rest is coupled with the gastric rhythm in an extended cortical network (Rebollo, Devauchelle, Béranger, & Tallon‐Baudry, 2018), including known viscero‐sensitive regions such as primary and secondary somatosensory cortices, as well as the parieto‐occipital region where gastric‐alpha coupling was observed. Gastric‐brain coupling thus appears to play a role in the large‐scale organization of brain dynamics at rest. Although little employed so far, the EGG thus appears as an attractive method to study brain‐viscera interactions.
1.3. Physiological basis of the EGG
1.3.1. Pacemaker cells and smooth muscles
The stomach mixes ingested food with secretions and grinds it into particles that can be emptied into the duodenum through the pylorus (Figure 2a). The stomach is classically divided into two parts (Koch & Stern, 2004). The proximal stomach consists of the fundus (upper curved part) and the corpus (or body, the main central part of the stomach), which acts as a reservoir and controls intragastric pressure (Tack, 2012). The distal stomach consists of the lower part of the corpus, the antrum, and the pylorus, and is responsible for the mixing, grinding, and emptying of solid food (Kelly, 1980; Rayner, Hebbard, & Horowitz, 2012). The gastrointestinal tract contains two muscular layers that control gut peristalsis: a thin outer longitudinal layer and a thick inner circular layer. Unlike the rest of the organs of the gastrointestinal tract, the stomach has an additional innermost oblique layer of smooth muscles, which allows more refined control of motility patterns (Birmingham, 1898; Christensen & Torres, 1975; Fritsch & Kühnel, 2008).
Figure 2.
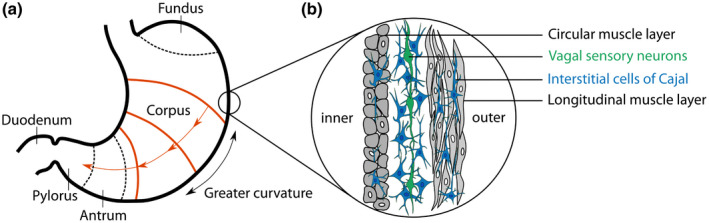
The stomach and the generation of the gastric slow rhythm. (a) Anatomical regions of the stomach, with the main divisions into fundus, corpus, and antrum. The gastric rhythm originates from the pacemaker region (orange) near the greater curvature of the mid/upper corpus. From here, it entrains other pacemaker cells, resulting in traveling rings of electrical wavefronts in the direction of the antrum (O’Grady et al., 2010). (b) The Interstitial Cells of Cajal (ICC, blue) are the generators of the gastric rhythm. They lay in the stomach wall, between and within the circular and longitudinal muscle layers. An additional thin oblique muscle layer located in the innermost part of the stomach, adjacent to the circular layer, is not represented here. The electrical activity of the pacemaker is passed through the entire ICC network and is also passively conducted into coupled muscle cells. ICCs make synapse‐like contact with vagal sensory neurons (Powley et al., 2008), presented in green, in a structure known as intramuscular arrays, that can detect mechanical changes in smooth muscles. Adapted from Koch & Stern, 2004
The stomach wall contains a distinctive type of cells, the Interstitial Cells of Cajal (ICCs), located between the circular and longitudinal muscular layer (myenteric Interstitial Cells of Cajal, ICC‐MY) or within the muscular layers (intramuscular Interstitial Cells of Cajal, ICC‐IM) (O’Grady, 2012; Sanders, Ward, & Koh, 2014) (Figure 2b). Although ICCs are not neurons (Klüppel, Huizinga, Malysz, & Bernstein, 1998), they display neuron‐like properties. Both types of ICCs continuously and intrinsically generate and propagate slow pacemaker currents (for review see Huizinga & Chen, 2014), constituting the basis of the gastric rhythm (Hirst & Edwards, 2006; Sanders, Koh, & Ward, 2006; Sanders et al., 2014). During digestion, the gastric rhythm generated by ICCs triggers smooth muscle contraction with additional inputs from excitatory enteric motor neurons (Sanders et al., 2014) and vagal efferent neurons (Chang, Mashimo, & Goyal, 2003). In addition to their role in slow wave generation, ICC‐IM is involved in the transduction of inputs from enteric motor neurons (Hirst & Edwards, 2006; Sanders et al., 2006, 2014). While ICCs control the pace of gastric contractions, enteric motor neurons and vagal efferent neurons regulate the amplitude of contractions. It follows that the frequency of the surface EGG is likely to be related to the intrinsic pacemaker activity of ICCs, while EGG amplitude is related to a combination of currents generated in ICCs, enteric motor neurons, and smooth muscles.
ICCs generate the gastric rhythm and actively propagate the slow waves through the ICC network as well as to electrically coupled smooth muscle cells. Rings of electrical wavefronts travel circumferentially in a proximal to distal gradient along the stomach (Koch & Stern, 2004), pushing food toward the pylorus when accompanied by muscular contractions. How wave propagation is orchestrated is not known with certainty. It has since long been assumed that the stomach contains a “dominant pacemaker” area in the greater curvature of the mid/upper corpus, entraining slow waves at other sites, possibly with a gradient in frequency (Hinder & Kelly, 1977; Kelly, Code, & Elveback, 1969; Koch & Stern, 2004; O’Grady et al., 2010; Riezzo et al., 2013).
1.3.2. Relating cutaneous EGG to gastric physiology
Because the frequency of the gastric rhythm is determined by ICCs’ intrinsic pacemaker activity, cutaneous EGG frequency directly reflects the frequency of the gastric basal rhythm, as revealed by simultaneous cutaneous EGG and invasive recordings in humans (Brown, Smallwood, Duthie, & Stoddard, 1975; Chen, Schirmer, & McCallum, 1994; Coleski & Hasler, 2004; Familoni, Kingma, & Bowes, 1987; Hamilton, Bellahsene, Reichelderfer, Webster, & Bass, 1986; Lin, Chen, Schirmer, & McCallum, 2000; Mintchev, Kingma, & Bowes, 1993).
The relative contribution of ICCs and smooth muscle contractions to cutaneous EGG amplitude is more difficult to estimate (Angeli et al., 2013; Bayguinov, Hennig, & Sanders, 2011; Hocke et al., 2009; O’Grady, 2012; Stern et al., 2007; Xing, Qian, & Chen, 2006), for two main reasons: First, the electrophysiological signature of smooth muscle contraction is filtered out in cutaneous EGG (Verhagen et al., 1999), and more generally how electrical signals of gastric origin are combined in surface recordings remains to be fully understood (Cheng, Du, & O’Grady, 2013; Du, O’Grady, Cheng, & Pullan, 2010). Second, the amplitude of the gastric rhythm is dependent on the fasting/fed state of the stomach. As a first approximation, one could consider that during digestion the EGG corresponds to a combination of muscle contractions and ICC intrinsic activity, whereas in the fasting state, the stomach is empty and surface EGG mostly corresponds to ICC activity (Smout, Van Der Schee, & Grashuis, 1980). This is the rationale underlying the clinical test comparing pre‐ and postprandial EGG amplitude. However, even when the stomach is resting as in moderate fasting, a few muscular contractions may occur (O’Grady et al., 2010; Sanders et al., 2014), and occasional intense muscular activity can be observed in prolonged (i.e., overnight) fasting (Koch & Stern, 2004).
Because ICCs are present all along the gastrointestinal tract, cutaneous electrodes might capture the myoelectrical activity of other organs of the GI tract, raising the question of the organ‐specificity of the signal. The small intestine displays frequencies that are much higher than the stomach, usually above 0.16 Hz (Christensen, Schedl, & Clifton, 1966; Riezzo et al., 2013; Waldhausen, Shaffrey, Skenderis, Jones, & Schirmer, 1990). The frequency range of the colon is broader, ranging from 2 to 12 cycles per minute in humans (Erickson et al., 2019; Homma et al., 1995; Pezzolla, Riezzo, Maselli, & Giorgio, 1989; Riezzo, Pezzolla, Maselli, & Giorgio, 1994; Taylor, Duthie, Smallwood, & Linkens, 1975), that is, potentially overlapping in frequency with the gastric rhythm (Amaris, Sanmiguel, Sadowski, Bowes, & Mintchev, 2002; Erickson et al., 2019). Still, numerous studies found that the 3 cpm rhythm disappeared, or was largely reduced, following surgical removal of the stomach but not of the colon (Homma et al., 1995; Imai & Sakita, 2005; Kaiho et al., 2000; Pezzolla et al., 1989).
1.4. Pathways of gut‐brain signaling
There is evidence in the cognitive neuroscience literature that stress or emotions can alter cutaneous EGG frequency or amplitude (Baldaro et al., 1996; Gianaros et al., 2001; Lin et al., 2007; Muth et al., 1999; Stern et al., 1991; Vianna et al., 2006), indicating descending influences from brain to stomach, and that ascending influences, from stomach to brain, influence brain dynamics (Richter et al., 2017). What are the currently known anatomical pathways supporting those interactions? In this section, we present the mechanisms of sensory transduction of the gastric rhythm and ascending pathways up to cortical targets, followed by an overview of descending projections from brain to stomach. Note that much remains to be determined, from signal transduction (Umans & Liberles, 2018) to anatomo‐functional pathways (Azzalini et al., 2019). Only very few stomach‐specific anatomical tracing studies exist in animals (for a recent example in rodents see Han et al., 2018). Besides, it is tempting to extrapolate from anatomical tracing and/or electrophysiological studies in animals (rodents, cats and monkeys) to humans, on the assumption that visceral pathways are probably ancient and conserved through evolution. However, differences between species have been reported (Bishop, Malliani, & Thorén, 1983; Pritchard, Hamilton, & Norgren, 2000; Shipley & Sanders, 1982). The overall description presented in this section is drawn from studies in rodents, cats and monkeys, and some pathways may differ in humans.
1.4.1. Detection of the gastric rhythm and mechanical changes in sensory neurons
The EGG reflects a combination of gastric smooth muscle contractions and of the gastric rhythm generated by ICCs. Both types of signals might be detected in the stomach by sensory neurons. ICCs make direct synapse‐like contact with vagal afferent neurons, also known as intramuscular arrays (Powley & Phillips, 2011; Powley et al., 2008). The gastric rhythm might thus be directly relayed to the brain through vagal afferent neurons, although this has not been directly tested. Experimental work has mostly been devoted to the signaling of gastric smooth muscle contractions. Multiple cell types, including ICCs, are grouped in arborized structures that run along the smooth muscle and act as mechanoreceptors across the gastrointestinal tract, with different sensitivity thresholds and adaptation profiles (Berthoud, Blackshaw, Brookes, & Grundy, 2004; Blackshaw, Brookes, Grundy, & Schemann, 2007; Umans & Liberles, 2018). Those mechanosensory structures continuously sense the contractile state of the stomach and can transmit changes in smooth muscles to the brainstem through vagal and spinal afferent fibers. It is worth underlining that in the vagus nerve, around 80% of the fibers are ascending, indicating the brain is probably more of a listener than a sender of vagal information (Agostoni, Chinnock, De Daly, & Murray, 1957). In contrast, the ratio between efferents and afferents in the spinal splanchnic nerve is closer to 50:50 (Foley, 1948; Leek, 1972).
1.4.2. Vagal and spinal pathways relay gastric information to the brainstem, thalamus, and cortex
Vagal sensory neurons project to the nucleus of the solitary tract in the brainstem, an important relay center for visceral information, that is also involved in the initiation of gastric control reflexes (Azpiroz & Malagelada, 1990). The nucleus of the solitary tract displays a rough viscerotopic organization (Altschuler, Bao, Bieger, Hopkins, & Miselis, 1989), but with local overlap between inputs from the heart and the gastrointestinal tract (Paton & Kasparov, 2000). Visceral afferents are relayed to the parabrachial nucleus (Figure 3, right panel), which integrates vagal and spinal information and is the main relay of visceral information to subcortical and cortical structures (Hylden, Hayashi, Bennett, & Dubner, 1985; Norgren, 1978; Pritchard et al., 2000).
Figure 3.
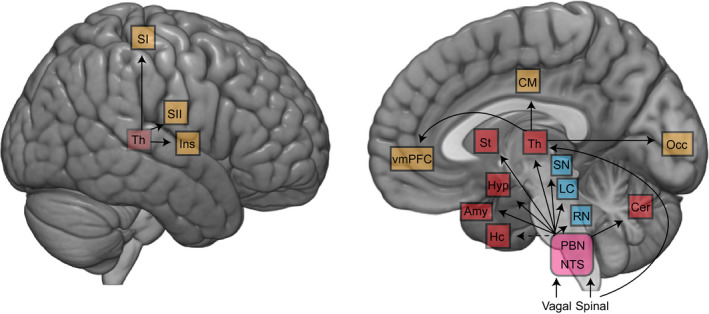
Projections of vagal and spinal afferents from the gastrointestinal tract to the brain. Afferents target brainstem nuclei (purple) including nucleus tractus solitarius (NTS) and parabrachial nucleus (PBN). The NTS and PBN in turn project to various subcortical structures, including the neuromodulatory structures (blue), as well as subcortical (red) and cortical (yellow) regions. Another spinal afferent pathway bypasses the brainstem and directly targets the thalamus. Abbreviations: Amy, amygdala; Cer, cerebellum; CM, cingulate motor regions; Hc, hippocampus; Hyp, hypothalamus; Ins, insula; LC, locus coeruleus; NTS, nucleus of the solitary tract; PBN, parabrachial nucleus; RN, raphe nucleus; SI, primary somatosensory; SII, secondary somatosensory; SN, substantia nigra; St, striatum; Th, thalamus; vmPFC, ventromedial prefrontal cortex. Modified from Azzalini et al., 2019
The nucleus of the solitary tract and parabrachial nucleus directly targets the main neuromodulatory centers (Figure 3): the serotoninergic dorsal raphe nucleus, the noradrenergic locus coeruleus, and the dopaminergic substantia nigra and ventral tegmental area (Coizet, Dommett, Klop, Redgrave, & Overton, 2010; Pritchard et al., 2000; Saper & Loewy, 1980). The functional relevance of gastric vagal signaling on the dopaminergic reward pathway has been recently elegantly demonstrated in mice (Han et al., 2018), where stimulation of the vagal sensory ganglion activated self‐stimulation behavior, conditioned place preferences, and induced dopamine‐release from substantia nigra. The parabrachial nucleus also targets the amygdala, the hypothalamus, and the striatum (Bester, Besson, & Bernard, 1997; Fulwiler & Saper, 1984; Saper, 2002).
Gastrointestinal inputs can reach the thalamus through parabrachial projections or direct spinothalamic pathways. Parabrachial outputs target the ventromedial, reticular, intralaminar, and ventroposterior thalamic nuclei (Coen, Hobson, & Aziz, 2012). Spinal and vagal inputs are already combined in the parabrachial nucleus and further convergence takes place in the thalamus. Unexpectedly, the lateral geniculate nucleus, a visual thalamic relay, receives massive inputs from the parabrachial region (Erişir, Van Horn, & Sherman, 1997; Uhlrich, Cucchiaro, & Sherman, 1988), and parabrachial activation affects visual responses in the lateral geniculate nucleus (Lu, Guido, & Sherman, 1993; Uhlrich, Tamamaki, Murphy, & Sherman, 1995) and cortex (Munk, Roelfsema, König, Engel, & Singer, 1996).
From the thalamus, numerous cortical areas receive visceral inputs (Figure 3), including primary and secondary somatosensory cortex (Amassian, 1951; Downman, 1951), insula (Cechetto & Saper, 1987), ventromedial prefrontal cortex (Vogt & Derbyshire, 2009) and cingulate motor regions (Dum, Levinthal, & Strick, 2009). In rodents, viscerotopy is present in the ventrobasal nucleus of the thalamus and in the insula (Cechetto & Saper, 1987). Although it is known that in humans the somatosensory cortex is coupled with the stomach (Rebollo et al., 2018) and that it responds to heartbeats (Kern, Aertsen, Schulze‐Bonhage, & Ball, 2013), whether the somatosensory cortex shows a viscerotopic organization, and how viscerotopy is integrated with somatotopy, has not between investigated since the 50’s (Downman, 1951).
1.4.3. Descending influences
As reviewed in Section 1.2, gastric amplitude and/or frequency can be modified by cognitive and emotional factors. Indeed, gastrointestinal functioning is regulated by both vagal (Hall, el‐Sharkawy, & Diamant, 1986; Stern, Crawford, Stewart, Vasey, & Koch, 1989), or parasympathetic, and spinal, or sympathetic, centers. The main parasympathetic center is the dorsal motor nucleus of the vagus (Gillis, Quest, Pagani, & Norman, 1989), that has descending projections to smooth muscle cells as well as to Interstitial Cells of Cajal (Schemann & Grundy, 1992; Travagli, Hermann, Browning, & Rogers, 2006). The vagal innervation from the dorsal nucleus of the vagus modulates the amplitude of the gastric rhythm and can have either an activating or inhibiting effect (Andrews & Scratcherd, 1980; Pagani, Norman, Kasbekar, & Gillis, 1985; Travagli et al., 2006). The sympathetic efferent nuclei controlling the stomach are located in the intermediolateral cell column of the thoracic‐lumbar spine, and project to prevertebral and paravertebral ganglia located outside the spine (Furness, 2006). In turn, spinal projections innervate enteric neurons, arterioles of the gut wall and striate muscles of sphincters to control vasoconstriction, liquid balance, secretion, blood flow, and motility (Furness, 2012; Holzer, 2006; Sveshnikov, Smirnov, Myasnikov, & Kuchuk, 2012). Sympathetic projections can induce either an inhibition or a stimulation of stomach contractions (Smirnov & Lychkova, 2003; Sveshnikov et al., 2012). Sympathetic projections are modulated by higher level structures, including parabrachial nucleus, nucleus of the solitary tract (Saper & Loewy, 1980), rostroventrolateral medulla (Deuchars & Lall, 2015), raphe nucleus (Morrison, Sved, & Passerin, 1999), locus coeruleus (Bruinstroop et al., 2012) as well as several hypothalamic nuclei (Deuchars & Lall, 2015).
1.4.4. Brain‐stomach coupling in humans
In humans, pioneering studies identified brain regions coupled with the stomach using gastric distension, induced by inserting and inflating a balloon in the stomach of participants, or, alternatively, by asking participants to drink a specific amount of liquid. Water ingestion can also be used to measure explicit gastric interoception, as recently proposed by van Dyck et al., 2016. Gastric distension activates somatomotor regions, insula, vmPFC and mid‐cingulate, and deactivates occipital regions (Ladabaum et al., 2001; Lu et al., 2004; van Oudenhove et al., 2009; Vandenbergh et al., 2005; Wang et al., 2008). More recently, stomach‐brain coupling was investigated during the resting state, without gastric stimulation. Rebollo et al., 2018, recorded the EGG in healthy participants during quiet rest, while simultaneously recording brain activity with functional magnetic resonance imaging. They then identified the regions where spontaneous fluctuations in the BOLD signal were phase‐synchronized with the gastric rhythm. This revealed an extended network including primary and secondary somato‐sensory cortices, mid‐cingulate areas, and extended portions of the occipital lobe, indicating that gastric‐brain coupling contributes to the large‐scale organization of brain activity at rest.
2. THE EGG: RECORDING, PREPROCESSING, AND DATA QUALITY ASSESSMENT
The aim of this section is to propose practical suggestions to record the EGG in the typical young and healthy population sampled in psychophysical research, as well as a semi‐automatized procedure to assess data quality and to extract the gastric rhythm characteristics in terms of amplitude, frequency, and phase. The procedure aims at identifying the gastric rhythm, that is, a regular oscillation in the normogastric range (2–4 cpm). Such a signal can safely be attributed to the stomach, whereas artifacts are likely to disrupt the regularity of the rhythm. It follows that the procedure as it currently stands is not appropriate to investigate departure from normogastria. The procedure reported here is also not designed to analyze the spatial propagation of the gastric waves along the stomach, and we refer the interested reader to Angeli et al., 2015; Bradshaw et al., 2016; Gharibans, Coleman, Mousa, & Kunkel, 2019; O’Grady et al., 2010, 2012.
2.1. Recording apparatus
The EGG is recorded with standard cutaneous electrodes, similar to electrocardiogram electrodes, and standard skin preparation (e.g., slight abrasion of the skin for optimal skin‐to‐electrode interface contact). EGG amplitude lies typically between 50 and 500 microVolt, commensurate with electroencephalography (EEG). Standard EEG acquisition systems can thus adequately amplify EGG signals, but several additional conditions must be met, due to the very slow pace (~0.05 Hz) of the gastric rhythm. First, DC amplifiers are best suited to record the EGG since even a very low high‐pass filter might distort the data. Most recent EEG acquisition systems have the large analog‐digital conversion range required for DC recordings without amplifier saturation. Second, recordings have to be long enough to collect a sufficient number of gastric cycles. As a rule of thumb, one minute contains about three gastric cycles, and 15 min correspond to only 45 cycles. Note that it can be useful to acquire some extra data (about 40 s) before and after the period of interest to facilitate off‐line filtering at the very low frequency of the gastric rhythm. Last, sources of very slow fluctuations in the recordings have to be minimized. In particular, hanging wires might swing around and induce slow drifts in the recordings and should thus be taped to a fixed support. Wrapping the wires in a shield can limit wire swinging as well as reduce electromagnetic artifacts. Because EGG frequency is around 0.05 Hz, sampling frequency could in principle be very low (below 1 Hz). However, a higher sampling frequency is required for proper artifact identification, in particular, participant's movements that are accompanied by muscle artifacts.
2.2. Participants
2.2.1. Participants’ information and inclusion
Participants are informed early in the inclusion process of electrode location, which implies that their shirt is raised, their skin exposed between navel and sternum, and shaved if too hairy. Participants feeling uncomfortable with the procedure can thus withdraw at that early stage, and are informed that they can withdraw at any time later on. Participants might also feel more comfortable if the experimenter placing the electrodes is of the same gender as the participant. Last, participants are asked to avoid tight‐fitting clothes that might potentially touch the electrodes and hence compromise recording quality.
Participants should obviously have no gastric or digestive disorder. Several medications might influence the EGG, including prokinetic anti‐emetic agents, narcotic analgesics, anticholinergic drugs, and anti‐inflammatory agents, as well as probiotics and prebiotics (Américo, Miranda, Corá, & Romeiro, 2009; Chiba et al., 2007; Indrio et al., 2009; Walldén, Lindberg, Sandin, Thörn, & Wattwil, 2008). Different recommendations exist in the gastroenterology literature with regards to the inclusion of participants taking these medications (Murakami et al., 2013; Riezzo et al., 2013; Yin & Chen, 2013). A practical solution for psychophysiological studies in healthy participants is to include only subjects without medication.
Another inclusion/exclusion criterion that might prove useful is the body mass index (BMI, Weight (kg)/ (Height (m))2). Participants with a high BMI typically display a lower EGG amplitude (Riezzo, Pezzolla, & Giorgio, 1991; Simonian et al., 2004; Somarajan, Cassilly, Obioha, Richards, & Bradshaw, 2014), potentially because a thicker abdominal wall increases the distance between the electrical source and the recording electrodes and hence results in a lower amplitude recording (Liang & Chen, 1997; Obioha et al., 2016). In addition, people with high BMI show more activity outside the 2–4 cpm range (McCallum, Jones, Lin, Sarosiek, & Moncure, 2001; Simonian et al., 2004; Tolj, 2007), and in morbid conditions, altered gastric emptying (McCallum et al., 2001; Tosetti et al., 1996). In sum, it thus might prove advantageous to include only rather lean participants. In practice, we include subjects with a BMI comprised between 18 and 26. Note that the phase of the menstrual cycle might impact EGG frequency (Parkman et al., 1996; Tolj, 2007) and should thus be documented if the absolute EGG frequency is a parameter of interest.
Studies in gastroenterology focus on the typical EGG amplitude increase following food ingestion, reflecting muscle contractions and gastric motility and other parameters (Stern, Jokerst, Livine, & Koch, 2001; Stern et al., 1989). Another option for psychophysiology is to ask participants to fast for at least 2 hr preceding their appointment (i.e., about 3 hr before the actual beginning of the recording) to focus the analysis on the basal gastric rhythm in an almost empty stomach with little muscular contractions. Only about 10% of a solid meal remains in the stomach 2 to 3 hr after meal ingestion (Vasavid et al., 2014). In any case, the time elapsed since the last meal should be documented and taken into account in the experimental design. Note that details on the contents of pre‐fast meal and feeling of hunger at the time of recordings are potentially relevant parameters but were not recorded in this data set.
2.2.2. Participants’ position
Most studies in gastroenterology record EGG with the patient in a lying position, which reduces voluntary movements, although ambulatory EGG recordings are currently being developed (Gharibans et al., 2018). Another advantage of a lying position is that the electrodes rest on the abdominal surface and not in fatty folds (Koch & Stern, 2004). However, a good EGG can also be obtained in a sitting position, although the amplitude is typically a bit lower, most likely due to the distance of the stomach to the skin surface (Jonderko, Kasicka‐Jonderko, & Blonska‐Fajfrowska, 2005). In practice, we place the electrodes while subjects are in a standing or lying position, thus allowing easier access to anatomical landmarks. After electrodes are placed, we record from participants in a semi‐reclined sitting position, as is customary for MEG or EEG recordings, and ask participants to avoid any voluntary movement. Movements induce large artifacts in the recording, and artifacted data segments should be identified and excluded from further analysis (Verhagen et al., 1999).
2.3. Electrode placement
Unlike electrocardiography, electrode placement in electrogastrography is not standardized. The stomach is located in the upper left abdomen but the precise location with respect to external landmarks varies (Gharibans et al., 2019). Many clinical studies used between 2 and 4 electrodes. We initially used a 17 electrode grid (Richter et al., 2017), which proved too large, and after some trials and errors found that a grid of seven electrodes provided sufficient coverage to detect a good quality EGG in most participants. The coverage we propose is sufficient to detect a good quality signal in at least one recording location; if the aim of the study is to analyze the propagation of the gastric rhythm along the stomach, a higher density of electrodes and a wider coverage is recommended (Gharibans et al., 2019). Recording locations are illustrated in Figure 4a. The electrode location proposed here deviates from the electrode location reported in the clinical literature (see for instance the review of Riezzo et al., 2013) in that it covers lower portions of the abdomen. In clinical settings, electrodes are most often located in the vicinity of electrodes 2, 3, 5, and 7 in Figure 4 (Chen et al., 1999; Geldof et al., 1989; Koch & Stern, 2004; Mintchev et al., 1993; Parkman et al., 2003; Simonian et al., 2004).
Figure 4.
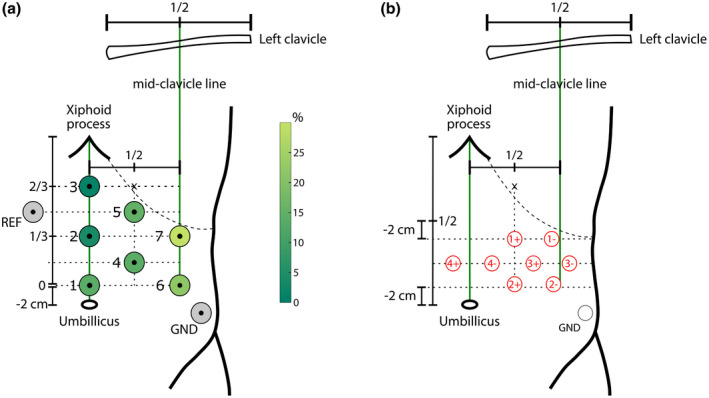
Localization of electrodes with respect to anatomical landmarks (umbillicus, xiphoid process, mid‐clavicular line, and coastal margin). (a) Setup for a unipolar montage. The circle area and color code at each electrode location indicate how often this electrode was found to display the largest gastric rhythm, in a sample of 100 healthy participants with a good spectral signature of the gastric rhythm. (b) Setup for a bipolar montage, better suited for fMRI recordings. See text for detailed explanations. REF: Reference. GND: Ground
In the proposed setup (Figure 4a) the first electrode (1) is placed 2 cm above the umbilicus. Electrodes 2 and 3 are placed above electrode 1 on the midline, at, respectively, one‐third and two‐thirds of the distance between electrode 1 and the xiphoid process. The locations of two other electrodes (6, 7) are determined by a vertical line crossing the midpoint of the left clavicle, and horizontally by the locations of electrodes 1 and 2. Note that the electrode 7 location might fall above the rib cage, in which case it is advisable to shift it toward the midline to improve the signal to noise ratio. Finally, electrodes 4 and 5 are placed in between the two columns (1, 2, 3) and (4, 5), at the level of the vertical midpoint between electrodes 1 and 2 and electrodes 2 and 3. The reference electrode is placed symmetrically to electrode 5. Finally, the ground electrode is placed over the left abdomen, above the iliac crest. This setup can be combined with EEG and/or MEG recordings.
The EGG can also be recorded in an MRI environment, but it might require the use of bipolar electrodes to avoid amplifier saturation. We used the following scheme (Figure 4b; Rebollo et al., 2018): Four bipolar electrodes are placed in three rows over the abdomen, with the negative derivation placed 4 cm to the left of the positive one. The midpoint between the xiphoid process and umbilicus is identified, and the first electrode pair is set 2 cm below this area, with the negative derivation (1−) set at the point below the rib cage closest to the left mid‐clavicular line. The second electrode pair (2+, 2−) is set 2 cm above the umbilicus and aligned with the first electrode pair. The positive derivation of the third pair (3+) is set in the center of the square formed by electrode pairs one and two. The positive derivation of the fourth electrode pair (4+) is centered on the line traversing the xiphoid process and umbilicus at the same level as the third electrode. The ground electrode is placed above the iliac crest (Figure 4b). Note that scanner artifacts are much faster than the gastric rhythm and can easily be filtered out, at least with a standard echo‐planar imaging sequence and that the B0 magnetic field of the scanner does not affect EGG frequency (Rebollo et al., 2018).
2.4. Power spectrum and channel selection
The first step in data analysis is to extract the gastric rhythm. We first present processing steps for a good quality recording and come back to noisy data and artifacts in section 2.6. Spectral power at each electrode is computed to identify the location with the largest activity in the normogastric 0.033–0.066 Hz (2–4 cpm) range and to determine the peak frequency of each participant. Several methods for spectral power estimation can be used. Here, we used a Fast Fourier Transform (FFT) as implemented in the Fieldtrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011) with a Hanning taper to reduce spectral leakage and control frequency smoothing. We provide the relevant code for spectral estimation and other analyses at https://github.com/niwolpert/EGG_Scripts (for computing the power spectrum, see function “compute_FFT_EGG”). As illustrated in Figure 1c, a good quality recording shows a distinctive spectral signature in the normogastric range, with a peak frequency similar at most, if not all, recording sites. The channel with the largest power at peak frequency is selected for further analysis. Note that the spectral signature of the EGG is clearly different from the spectral signatures of either respiration or heartbeats (Figure 1d), and that a harmonic at twice peak frequency might be observed. Peak frequency is usually fairly stable over a couple of hours but might vary over longer recording times (Lindberg, Iwarzon, & Hammarlund, 1996). Power and amplitude might also be extracted from the spectral analysis.
2.5. Phase and amplitude of the filtered signal
For a more refined, time‐resolved analysis of the EGG, the next step is to filter the raw EGG from the selected channel around the participant's peak frequency to better isolate the gastric rhythm. Several types of filters might be considered, bearing in mind that the very low frequency of the EGG imposes additional constraints on filter design and filter stability. We opted for a finite impulse response filter, known to be more stable and less likely to introduce nonlinear phase distortions (Cohen, 2014), and more precisely a third‐order frequency sampling designed finite impulse response filter (MATLAB: FIR2), with a bandwidth of ±0.015 Hz around the participant's peak EGG frequency (function “compute_filter_EGG”). For instance, if peak frequency is exactly 0.05 Hz (3 cpm), the filter covers the range between 0.035 and 0.065 Hz (2.1 and 3.9 cpm). If peak frequency is 0.035 Hz (2.1 cpm), close to the lower limit of the normogastric range, the filter range is 0.02–0.05 Hz (1.2–3 cpm) and therefore also includes signal outside the normogastric range. Of note, filtering, especially at low frequencies, is difficult, and the actual filter deviates from the ideal filter, with smoother transitions, extending at higher and lower frequencies, as illustrated in Figure 6a. Filter width is designed to be wide enough to capture physiological fluctuations in the duration of the gastric cycle, but narrow enough to exclude not only respiration, but also the harmonic of the gastric rhythm (Verhagen et al., 1999).
Figure 6.
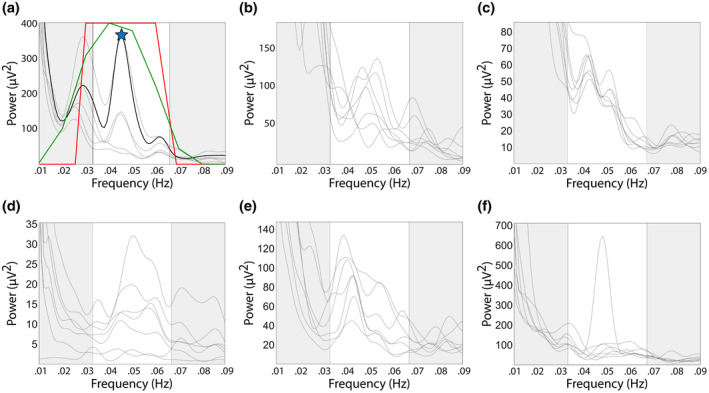
Examples of power spectra of data included in further analysis (a) or discarded (b‐f). Each line corresponds to a recording channel, and the spectral region highlighted in white corresponds to normogastria. (a) Power spectrum with a well‐defined spectral peak in the normogastric range, occurring in several channels at the same frequency. The star indicates peak frequency and the black line corresponds to the channel with the largest power at peak frequency. The red line represents the ideal filter, and the green line the best fit for the ideal filter. (b) Power spectrum with peaks at different frequencies in different channels. (c) Power spectrum with spectral peaks at two different frequencies. (d) Power spectrum with a broad peak, well defined in only one channel. (e) Several channels display a spectral peak but at different frequencies. (f) A well‐defined spectral peak is present, but only in one channel
By applying the Hilbert transform to the filtered data, we retrieve the instantaneous phase and amplitude envelope of the gastric rhythm. Figure 5 shows two examples of the filtered signal, and amplitude and phase obtained after applying the Hilbert transform. The distribution of cycle duration is usually Gaussian (Figure 5b), with sometimes outliers (Figure 5d), defined as exceeding mean ± 3 SDs. We observed that cycles with abnormally long or short duration also often presented a nonmonotonous phase evolution (inset in Figure 5c).
Figure 5.
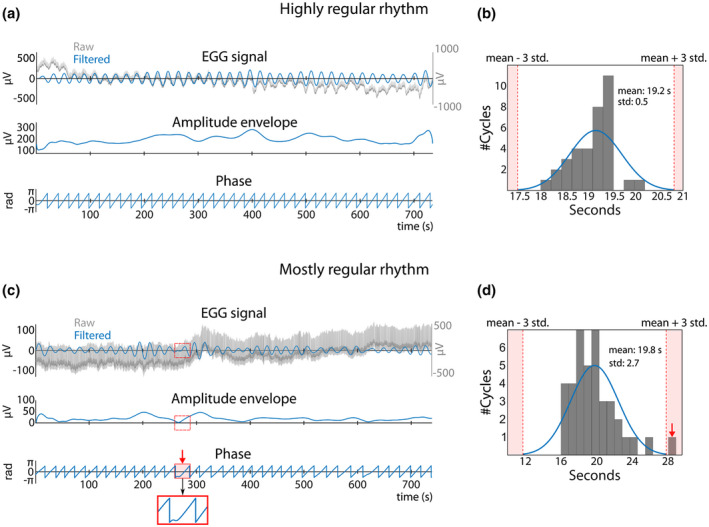
Two examples of EGG signal and corresponding amplitude and phase that reveal a highly regular rhythm (top) or a mostly regular rhythm (bottom). (a) Top row: Raw signal (grey) with superimposed filtered EGG (blue), obtained by filtering the raw signal ±0.015 Hz around the peak frequency of the recording. The Hilbert transform generates two time series: the amplitude envelope (middle row) and instantaneous phase of the gastric rhythm in radians (bottom row). (b) Distribution of cycle durations. Red dotted lines indicate mean cycle duration ± three SDs. In this example, the distribution of cycle duration is quite narrow, without any outlier. (c) Example of a different recording with mostly regular phase time series. The gastric rhythm is not always visible to the naked eye in the raw signal (top row) and its amplitude is sometimes very low (middle row). A cycle shaded in red and marked by a red arrow shows a nonmonotonous change in phase (bottom row) and concomitant low amplitude. (d) Histogram of cycle duration. The cycle with nonmonotonous change in phase in (c) appears as an outlier (red arrow). The cycle is considered as an artifact (nonmonotonicity and cycle duration) and therefore discarded from further analysis
2.6. Identification of noisy recordings
We have presented so far good quality recordings. Identifying noisy recordings, or noisy segments of data, is of course critical. In the following, we suggest some criteria to guide decisions.
2.6.1. Power spectra
The power spectrum is a good indicator of data quality. As shown in Figure 6a, a high‐quality recording shows a clear spectral peak in normogastric range with a peak frequency that is congruent across several channels. We systematically discarded power spectra with variability in peak frequency between electrodes (Figure 6b‐e). We also discarded cases where a clear spectral signature is observed, but at only one location, as in Figure 6f. As detailed in Section 3, the power at peak frequency (between 26 and 8,800 μV2 in our data) does not appear as a reliable indicator of signal quality.
2.6.2. Identification of participants with highly variable gastric cycle duration
Once the EGG is filtered at the selected channel around peak frequency, we do a second quality check based on the regularity of the cycle durations. We estimated, in each participant, cycle duration from the phase of the Hilbert transform and computed the SD of cycle duration (see function “compute_std_cycle_duration”). The distribution of the SD of cycle duration in the 100 participants we recorded is shown in Figure 7a. Four participants clearly lie outside the distribution and are considered outliers. We retained a criterion of the SD of cycle duration smaller than 6 to include participants in further analysis.
Figure 7.
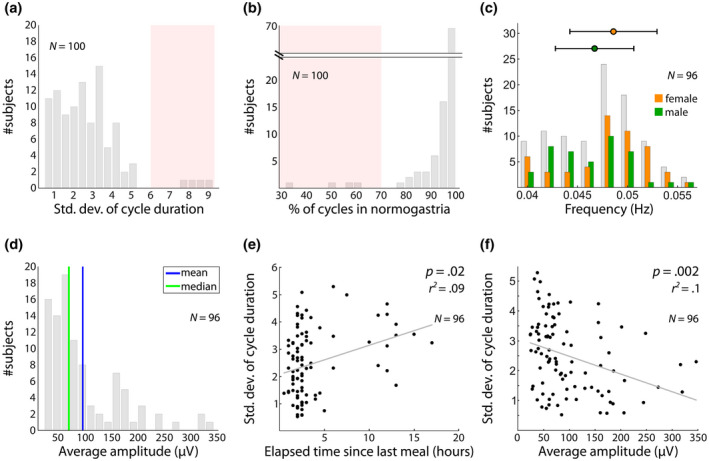
Distribution of EGG features across a sample of 100 young healthy participants. (a) Distribution of SDs of cycle duration. A cutoff at six SDs (red shaded area) isolates four outlier participants with more irregular cycles. (b) Percentage of cycles in normogastria (2–4 cpm). A cut‐off at 70% (red shaded area), as proposed by the clinical literature (Riezzo et al., 2013) isolates the same four outlier participants. (c) EGG peak frequency in the 96 remaining participants, with a mean of 0.048 Hz and SD of 0.004 Hz. Peak frequency is higher in female (M = 0.0486 Hz, SD = 0.0044) than male (M = 0.0467 Hz, SD = 0.0039) participants (rank sum test z = −2.25, Bonferroni‐corrected p = .15). Horizontal bars represent the SD. (d) Robust correlation between BMI and average amplitude. BMI shows no significant relationship with mean amplitude (Bonferroni‐corrected p = 1). (e) Robust correlation between elapsed time since the last meal and variability of cycle duration, expressed in SD around the mean for each participant. Longer fasting is associated with higher cycle irregularity (robust regression, Bonferroni‐corrected p = .02, r 2 = .09), an effect mostly driven by prolonged fasting (>10 hr). (f) Robust correlation between average amplitude and SD of cycle duration. There is a significant negative relationship, with higher amplitude being associated with lower variability of cycle duration (robust regression, p = .002, r 2 = .10)
We compared this data‐driven procedure with a procedure based on the clinical EGG literature (see function “show_prop_normogastria”), where it is held that EGG recordings in a healthy subject should be composed of at least 70% cycles in the normogastric range (2–4 cpm, or cycle duration between 15 and 30 s) (Parkman et al., 2003; Riezzo et al., 2013). Bradygastria refers to slow cycles ranging between 30 and 60 s (1–2 cpm) and tachygastria to short cycles between 6 and 15 s (4–10 cpm) (Riezzo et al., 2013). We found that those four participants identified as outliers in the distribution of SD of cycle duration (Figure 7a) are also outliers in the percentage of cycles in normogastria and exhibit less than 70% cycles in the normogastric range (Figure 7b). The two criteria thus appear equivalent in this data set.
Note that this processing step is dependent on the filter width (ideal and best fit) used. It is not suited for studies attempting to induce shifts in gastric peak frequency, resulting in a larger variability of gastric cycle duration or even in a shift of gastric peak frequency outside the normogastric range, as when eliciting nausea or disgust (Geldof et al., 1989; Harrison et al., 2010; Meissner et al., 2011; Stern et al., 1985).
2.7. Identification of artifacted data segments
Once recordings of overall good quality have been selected, artifacts transiently affecting the data have to be identified. Any type of artifact which involves a movement of the wires or of the abdominal wall might contaminate the signal. This includes for example movement of the legs, abdomen or torso, touching of the electrodes or wires, talking or coughing. Artifacts perturb the signal not only at the exact time of their occurrence, but spread over time given the very low frequency of the filter used.
The procedure we propose to identify artifacted data segments is based on two criteria: a cycle whose length exceeds the mean ±3 SDs of the cycle length distributions (Figure 5b, d), and a cycle that displays a nonmonotonic change in phase (inset in Figure 5c). This procedure is implemented in the function “detect_EGG_artifacts.” Any cycle meeting at least one of those criteria is considered as artifacted. Note that whether cycles tagged as artifacted by this procedure represent real artifacts or truly irregular gastric activity remains an open question.
It is advisable to complement the semi‐automated procedure we propose by a visual inspection to confirm artifact detection (Verhagen et al., 1999), but also to verify that the filtering, which can get unstable at very low frequencies, did not induce signal distortion.
2.8. Conclusion on preprocessing steps
We propose a preprocessing procedure of the EGG consisting of different steps with associated quality checks. All steps aim at identifying a regular rhythm, with a progression in the refinement of the analysis level. This procedure is summarized in Figure 8: In a first step, we decide whether the peak in the power spectrum is clear enough, with a peak frequency consistent between recording sites, to allow for the selection of a channel and a peak frequency. In a second step, the data are filtered and the phase computed, together with the distribution of cycle length. If at least 70% of the cycles lie in the normogastric range, and/or if the SD of cycle length is smaller than 6, the recording is considered to be of sufficient quality, else it is discarded. The last step consists of identifying artifacted data segments, based on cycle duration and phase monotonicity within a cycle.
Figure 8.
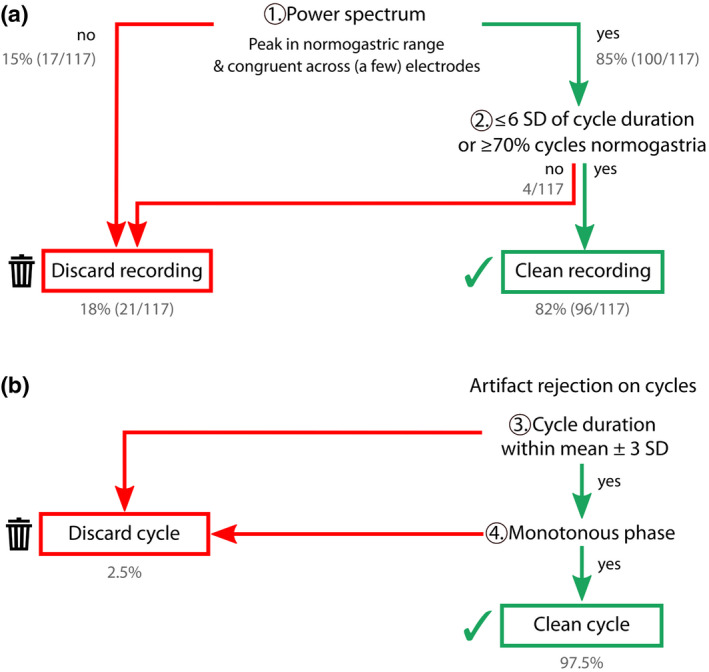
Decision tree with processing steps and corresponding criteria for a good quality recording/cycle. Grey numbers document the outcome of this procedure in a data set of 117 participants. (a) Decision tree for whether or not the recording can be retained for further analysis, depending on the presence of the spectral signature of the gastric rhythm and rhythm regularity. (b) Additional decision tree to detect artifacted cycles, based on the cycle duration and monotonicity of phase evolution
3. NORMATIVE DATA AND INTERINDIVIDUAL VARIABILITY IN YOUNG, HEALTHY VOLUNTEERS
Here, we apply the procedures described in Section 2 in a large data set of EGG recordings (N = 117) obtained in our group, from which normative parameters of the gastric rhythm in a healthy, young, and rather lean population can be derived.
3.1. Material
We used the data from 117 healthy participants (52 male, 65 female) sitting in a reclining chair. Participants were aged between 18 and 30 years (mean: 24, SD = 3.1) and had a BMI comprised between 16 and 26 (mean: 21.3, SD = 2.03). We aimed at recording participants in a moderate fasting state, where stomach contractions are scarce, and thus asked participants to fast for at least two hours before their appointment. One data set (N = 17) corresponds to the re‐analysis of published data (Richter et al., 2017), the rest corresponds to various unpublished pilot studies. Most participants (N = 75, including the participants from Richter et al., 2017) were recorded for 12–15 min at rest with eyes open using the MEG acquisition system of Elekta Neuromag® with a sampling frequency of 1,000 Hz, DC to 330 Hz. EGG data were also recorded in 25 participants performing an experiment on visual perception at a threshold for 12 min, and 17 subjects viewed a short movie (“Bang! You're Dead” from Alfred Hitchcock, 1961) during 15 min, using a BioSemi acquisition system with a sampling frequency of 2048 Hz, DC to 400 Hz. A subset of 66 participants filled out the Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). All participants signed a written informed consent and were paid for participation. The procedures were approved by the Ethics Committee CPP Ile de France III and were in accordance with the Helsinki declaration.
EGG was recorded as described earlier. We used the montage of seven electrodes described above, except in the 17 participants of Richter et al., 2017. Here, we had used a bilateral grid of 19 EGG electrodes (17 active, 1 reference, and 1 ground) placed over four regularly spaced rows, that we subsampled to match the current seven active electrodes schema.
3.2. Results
3.2.1. EGG identification in 117 recordings
We applied the procedures described above to the 117 EGG recordings (Figure 8). A well‐defined spectral peak within the normogastric range could be observed in 100 participants out of 117 (85%). The BMI of the 100 participants with an identifiable spectral peak (M = 21.2, SD = 2) was slightly, but significantly, lower than the BMI of the 17 participants where the peak could not be found (M = 22.3, SD = 2; t(115) = −1.99, p = .049). We then discarded participants whose gastric rhythm was irregular. Two different criteria could be considered, either a SD of gastric cycle duration larger than 6 (Figure 7a), or less than 70% of cycles in normogastria (Parkman et al., 2003; Riezzo et al., 2013). Those two criteria converged on the same four participants. Overall, our procedure was successful at recording and identifying the gastric rhythm in 82% (96/117) of the participants. Within the 96 remaining participants, 16 come from the study of Richter et al., 2017, and 80 from the new, unpublished data sets. Within the 96 recordings selected, 2.5% of the cycles were identified as artifacted, that is, as having an excessively long or short duration, or as displaying a nonmonotonous change in phase.
3.2.2. Electrode selection
For each participant, we identified, within the seven electrode grids, the electrode with the strongest peak in the 0.033–0.067 Hz range and selected it for all further analysis. The location of the selected electrode varied on a participant‐by‐participant basis, with electrode 1 selected in 14% of participants, electrode 2: 4%, electrode 3: 1%, electrode 4: 14%, electrode 5: 15%, electrode 6: 22%, electrode 7: 30%. Figure 4a shows, for each location in the grid, how often it was selected. The electrode showing the highest peak in the normogastric range was in the lower left abdominal region in more than half of the participants (electrodes 6 and 7) but all locations proved useful in at least one participant.
3.2.3. Analysis of gastric frequency, amplitude and cycle duration variability
We then analyzed several properties of the EGG in the 96 remaining participants. Mean dominant EGG frequency was 0.048 Hz (SD = 0.004) (Figure 7c). Female subjects had a slightly higher mean peak frequency than males, but this difference did not survive correction for multiple comparisons (Figure 7c; Mfemale = 0.0486 Hz, SD = 0.0044; Mmale = 0.0467 Hz, SD = 0.0039; rank sum test z = −2.25, uncorrected p = .03; Bonferroni‐corrected p = .15). As detailed in Table 1, we found no link between EGG peak frequency and age, BMI, anxiety, time of recording (morning versus. afternoon) or elapsed time since the last meal.
Table 1.
Descriptive statistics for EGG parameters and statistical relationships with sex, age, BMI, anxiety scores, daytime of recording and time from last meal
| Mean | Median | Min | Max | Gender (rank sum test) 43 male, 53 female | Age (robust correlation) N = 96 | BMI (robust correlation) N = 96 | Anxiety (robust correlation) N = 66 | Time of recording (rank sum test morning vs. afternoon) N = 100 | Elapsed time since last meal (robust corr.) N = 100 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Peak frequency (Hz) | 0.048 | 0.048 | 0.04 | 0.057 | p = .15 | r = −.06 | r = −.04 | r = .05 | p = 1 | r = −.07 |
| p = 1 | p = 1 | p = 1 | p = 1 | |||||||
| Average amplitude (μV) | 97.3 | 70.7 | 24 | 346.6 | p = 1 | r = .05 | r = −.14 | r = −.1 | p = 1 | r = −.06 |
| p = 1 | p = 1 | p = 1 | p = 1 | |||||||
| %normogastria | 96 | 98 | 79 | 100 | p = 1 | r = .02 | r = −.1 | r = .05 | p = 1 | r = −.22 |
| p = 1 | p = 1 | p = 1 | p = .22 | |||||||
| SD Dev cycle duration | 2.5 | 2.4 | 0.5 | 5.2 | p = 1 | r = −.06 | r = .14 | r = .11 | p = 1 | r = .3 |
| p = 1 | p = 1 | p = 1 | * p = .02 |
p values are Bonferroni‐corrected for the six tests performed. Significant results (corrected for multiple comparisons) appear in bold font.
Indicates Bonferroni‐corrected p < .05.
The mean average amplitude across participants was 97.3 μV (SD = 70.2) (Figure 7d). None of the demographical variables tested revealed any link with amplitude (Table 1), including BMI.
Last, we estimated fluctuations in EGG frequency by computing the SD of cycle duration. There was a significant positive correlation between longer fasting and higher variation in cycle length (Bonferroni‐corrected p = .02, r 2 = 0.09; Figure 7e), mostly driven by subjects with an elapsed time since last meal larger than 10 hr. Participants with a large EGG amplitude had more regular cycles, as revealed by the negative correlation between amplitude and SD of cycle length (p = .002, r 2 = 0.1; Figure 7f). None of the other variables measured co‐varied with the SD of cycle duration.
The data came from different experimental conditions (resting state, acquired with a BioSemi system, and various visual tasks, acquired with a BioMag system). No meaningful differences were found between experiments for any of the EGG parameters tested, apart from a difference in EGG amplitude, which might reflect more a difference in gain calibration between the two recording systems rather than on experimental conditions.
3.2.4. Amplitude in clean versus artifacted data segments
We finally compared EGG amplitude in “clean” versus artifacted cycles, that is, cycles that were either of extreme duration or nonmonotonous. No significant difference in amplitude was found between clean cycles (M = 96.6 μV, SD = 86.3) and cycles of extreme duration (M = 86 μV, SD = 51.2; rank sum test z = −.4, p = .67). Amplitude in nonmonotonous cycles (M = 40.3 μV, SD = 39) was significantly lower than in clean cycles (M = 96.6 μV, SD = 86.3; rank sum test z = −8.5, p < .001). However, as shown in Figure 9, only a small proportion (20%) of the cycles with very low amplitude were nonmonotonous, and a number of nonmonotonous cycles have a large amplitude.
Figure 9.
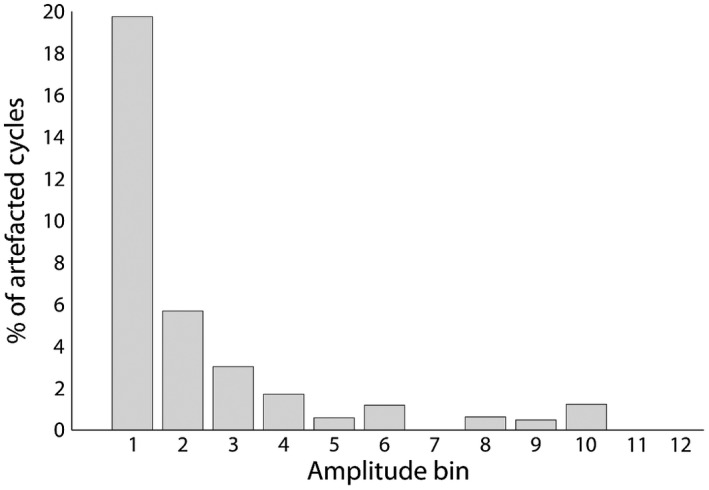
Percentage of EGG cycles classified as artifacted because of nonmonotonicity, as a function of EGG amplitude. Only 20% of the nonmonotonous cycles also have a very low amplitude
4. DISCUSSION
We propose here a procedure for recording and analyzing EGG data, and test this approach in 117 healthy, young, and rather lean male and female participants who had been fasting for at least two hours. The analysis pipeline aims at identifying a regular rhythm that can be safely attributed to the stomach, through multiple steps: by selecting only those participants who have a well‐defined spectral signature, with a peak frequency in the normogastric range (2–4 cpm) and regular cycles, and by excluding cycles whose duration exceeds mean ± 3 SD or whose phase is irregular.
We could identify the spectral signature of the gastric rhythm in 85% of the participants, most often at lower left abdominal locations. The largest amplitude could often be observed at electrodes located lower than usually recorded in clinical settings (Chen et al., 1999; Riezzo et al., 2013). Peak frequency was centered around 0.05 Hz, consistent with the gastroenterology literature, with a marginal difference in peak frequency between female and male participants. The large majority (96%) of the recordings with a clear spectral signature were also regular, with a SD of gastric cycle smaller than 6. The latter criterion proved equivalent to the criterion of 70% of cycles in the normogastric range (2–4 cycles per minute/0.033–0.067 Hz) employed in the clinical EGG literature. The parameter that most influenced the EGG was the time elapsed since last meal, with fasting longer than 10 hr leading to a more irregular rhythm. BMI, anxiety, and age had no noticeable relationship with EGG amplitude, frequency or regularity.
4.1. Electrode montage
We found that the sharpest spectral signature of the gastric rhythm could most often be found over lower left abdominal regions, that is, locations that are lower than in standard clinical settings (Riezzo et al., 2013; Simonian et al., 2004; Yin & Chen, 2013). This result is in line with recent EGG studies, where electrodes also covered a lower portion of the abdomen, but that were additionally informed, via CT scan analysis, on the precise stomach location and geometry in both patients and healthy controls (Gharibans, Kim, Kunkel, & Coleman, 2017, 2019). Both in the Gharibans et al. studies, and in ours, participants were seated, slightly or half‐reclined, thus differing from most clinical studies where a lying position is the norm (e.g., Geldof et al., 1989; Kaneoke et al., 1995; Lin, 1999), potentially leading to a different stomach position. In addition, we used here the reference electrode location commonly advocated in the clinical literature (Chen et al., 1999) over the upper right abdominal location, which might contribute to observing larger EGG amplitudes more often at the lower left location. While distance to reference electrode might contribute to EGG signal amplitude, it is unlikely to be the only determinant, for two reasons. In some participants, the largest EGG amplitude was detected close to the reference (i.e., electrodes 2 and 3). Conversely, the electrode where the largest EGG amplitude is most often detected (#7) is not the electrode the furthest away from the reference electrode. Note that reference‐free EGG data can be obtained by a higher density coverage and the surface laplacian transform (Gharibans et al., 2017).
Still, it is important to bear in mind that electrodes at lower locations might be more likely to record not only from the stomach, but also from other organs of the GI tract (Amaris et al., 2002; Erickson et al., 2019). While the rhythmic activity of the small intestine is at higher frequencies, the frequency range of the colon is broader, covering the frequency range between 0.03 and 0.13 Hz (Amaris et al., 2002; Erickson et al., 2019; Homma et al., 1995; Pezzolla et al., 1989; Riezzo et al., 1998), or even up to 0.2 Hz (Taylor et al., 1975). The frequency range of the colon and of the stomach might thus overlap, but the spectral signature of the stomach seems more narrow‐band than the spectral signature of the colon. It is thus important to couple the use of the low electrode montage we advocate with strict criteria to identify the spectral signature of the stomach in the recordings.
4.2. Data set selection based on spectral analysis and percentage in normogastria
The best evidence for gastric activity is a sharp spectral signature with a peak within the normogastric range. We retained two criteria: the sharpness of the spectral peak, and its presence at several recording locations. Applying those criteria in a rather strict manner led to discarding 18% of the participants, which is quite a large proportion, but this conservative approach should guarantee a large contribution of the gastric rhythm to the recorded signal. This first processing step could, and should, be improved in the future by finding a more quantitative approach to characterize the spectral signature.
The absence of the spectral signature of the gastric rhythm in EGG spectra can be attributed to various reasons. The signal might be too small because the stomach is too far away from the selected recorded locations, either because of an unusual stomach position or because abdominal fat increases the distance between stomach and electrode (Chen et al., 1999; Liang & Chen, 1997). Although participants with high BMI (above 26) are more likely to have more abdominal fat and to display a lower EGG amplitude (Riezzo et al., 1991; Simonian et al., 2004; Somarajan et al., 2014), we do not observe a link between EGG amplitude and BMI. However, participants without an EGG spectral peak had a higher BMI than participants with a spectral peak. This might indicate that abdominal fat can decrease the signal‐to‐noise ratio to the point that the gastric rhythm can no longer be detected. However, if the gastric rhythm can be detected, its amplitude and frequency do not depend on BMI, at least in the restricted BMI range explored in our sample. Signal to noise ratio might also be compromised because of artifacts, in particular, due to movement of the abdominal wall or of wires. Additionally, the gastric rhythm might be disorganized and hence display a blurred spectral signature, even in healthy participants which were screened for gastrointestinal pathologies.
After assessing power spectra, we quantified the regularity of gastric cycles using two independent approaches (SD of gastric duration smaller than 6 or 70% of cycles in normogastria), that converged and identified the same four participants with irregular EGG, out of 100. Note that the criteria on spectral signature are somewhat redundant with the criteria on cycle regularity. Indeed, irregular cycles are likely to result in a wide spectral peak, while we selected recordings with a well‐defined spectral signature.
4.3. Data segment selection
To the best of our knowledge, there is currently no standard for artifact rejection in EGG recordings, although some new methods are being developed for ambulatory recordings (Gharibans et al., 2018). Here, we chose to discard gastric cycles, that is, data segments of about 20 s, if the cycle was excessively long or short or if the phase displayed a nonmonotonous evolution within a cycle. Participants with a large EGG amplitude also had more regular cycles. At the single‐subject level, nonmonotonous cycles were more numerous when amplitude was low, but could be also observed in data segments with large amplitude. In other words, while there is a link between amplitude and cycle regularity, there is no one‐to‐one correspondence. It is important to underline that we do not know whether the cycles we discard represent artifacts (motion, electrical noise, …) accompanied with signal loss or a true irregularity of the gastric rhythm. Our procedure aims at extracting a regular rhythm that can be safely assumed to reflect the gastric rhythm. It is obviously unsuitable for clinical studies or cognitive studies in which irregularities of the gastric rhythm are of interest (see, e.g., Harrison et al., 2010).
4.4. Factors affecting EGG peak frequency, amplitude, and cycle duration variability
We found that women showed on average a slightly higher peaking frequency than men, a difference that was small and not strong enough to survive correction for multiple comparisons. This is in line with two previous studies (Parkman et al., 1996; Tolj, 2007) in comparably large sample sizes (N = 83 and N = 120). No effect was detected in smaller samples of adults (Pfaffenbach et al., 1995; Simonian et al., 2004) or in children (Riezzo et al., 1998). We did not document the phase of the menstrual cycle, which might impact EGG activity, although different studies yielded contradictory results (Parkman et al., 1996; Pfaffenbach et al., 1995; Tolj, 2007). Age was not related to EGG peak frequency, amplitude or cycle length variability, in line with previous studies investigating this age range (18–30 years) (Parkman et al., 1996; Pfaffenbach et al., 1995; Riezzo et al., 1991; Shimamoto et al., 2002; Simonian et al., 2004; Tolj, 2007). Note that our data set stems from a quite homogenous population: We did only include participants with a BMI range comprised between 18 and 26 and with an age between 18 and 30 years. EGG parameters are altered for higher values of BMI (Simonian et al., 2004; Tolj, 2007) or age (Parkman et al., 1996; Pfaffenbach et al., 1995; Riezzo et al., 1991; Shimamoto et al., 2002; Simonian et al., 2004; Tolj, 2007).
As time elapsed since the last meal increased, the EGG became more irregular. The correlation was mostly driven by participants having had their last meal more than 10 hr before the recording, that is, participants who had skipped breakfast. This suggests that in order to increase the chance of recording a regular signal, it might be recommended to ask participants to have a meal 2 to 4 hr before the recordings. This finding might potentially be linked to the observation that during very prolonged fasting (typically overnight), the stomach shows transient periods of strong contractions (also called the “phase III of the interdigestive complex”––Koch & Stern, 2004), which could impact the stability of the EGG. Combined with the classical finding that EGG amplitude increases right after a meal, these results emphasize the importance of taking into account the time elapsed since last meal in the experimental design (for instance by counterbalancing the order of presentation of different conditions between participants) and data analysis (for instance by adding time elapsed since last meal as a regressor).
Note that we restricted the analysis to a subset of EGG parameters (peak frequency, amplitude, cycle duration variability, percentage normogastria). Other parameters of interest, not studied here, are related to departure from normogastria (see e.g., Koch & Stern, 2004; Riezzo et al., 2013; Yin & Chen, 2013).
4.5. Conclusion: strengths and limitations
We propose here a full pipeline to record and analyze the EGG of young, healthy, and rather lean participants in a moderate fasting state, and validate it in a large data set. The pipeline aims at identifying a rhythm with a peak frequency between 0.033 and 0.067 Hz (2–4 cpm), and regular enough over time, so that it can safely be attributed to the stomach. It follows that we do not investigate lower or higher frequencies, for lack of criteria to discriminate signal from noise (Verhagen et al., 1999) and that the procedure we propose is not well suited for psychophysiological studies targeting large changes in gastric rhythm frequency, such as nausea, disgust, and stress. The procedure is also not suitable for investigating the spatial propagation of gastric slow wave along the stomach, and we refer the reader to other approaches (Angeli et al., 2015; Bradshaw et al., 2016; Gharibans et al., 2019; O’Grady et al., 2010, 2012).
The pipeline depends on the definition of the normal range of the gastric rhythm, an issue that is not fully resolved in the clinical literature (Chang, 2005; Parkman et al., 2003). This pipeline allows to estimate the duration of each gastric cycle, thereby providing a finer temporal resolution than approaches based on running spectral analysis (Stern et al., 2007). Gastric cycle duration estimation is dependent on the design and width of the filter used for analysis, which should be wide enough to capture physiological fluctuations of the gastric rhythm but narrow enough to exclude contaminating sources. Finally, the procedure is only semi‐automatized. Visual inspection is still required to detect large artifacts before any processing, to select power spectra satisfying all criteria, and to verify the quality of the filtering process.
CONFLICT OF INTEREST
The authors declare that no competing interests exist.
CUSTOM CODE
The custom code used for this article can be accessed online at the following address: https://github.com/niwolpert/EGG_Scripts
ACKNOWLEDGMENTS
We thank Margaux Romand‐Monnier, Clémence Almeras and Juliette Klamm for help during data acquisition. We also thank Ismael Jose Palacios‐García and Janina Hüer for helpful comments on an earlier version of the manuscript.
Wolpert N, Rebollo I, Tallon‐Baudry C. Electrogastrography for psychophysiological research: Practical considerations, analysis pipeline, and normative data in a large sample. Psychophysiology. 2020;57:e13599 10.1111/psyp.13599
Funding information
This work was supported by funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No 670325, Advanced grant BRAVIUS) and by a senior fellowship of the Canadian Institute For Advanced Research (CIFAR) program in Brain, Mind and Consciousness to C.T.‐B., as well as from ANR‐17‐EURE‐0017.
[Copyright line has been updated on 7 June 2020 after first online publication.]
REFERENCES
- Abell, T. L. , & Malagelada, J.‐R. (1988). Electrogastrography: Current assessment and future perspectives. Digestive Diseases and Sciences, 33(8), 982–992. 10.1007/BF01535995 [DOI] [PubMed] [Google Scholar]
- Agostoni, E. , Chinnock, J. E. , De Daly, M. B. , & Murray, J. G. (1957). Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. The Journal of Physiology, 135(1), 182–205. 10.1113/jphysiol.1957.sp005703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler, S. M. , Bao, X. M. , Bieger, D. , Hopkins, D. A. , & Miselis, R. R. (1989). Viscerotopic representation of the upper alimentary tract in the rat : Sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. The Journal of Comparative Neurology, 283(2), 248–268. 10.1002/cne.902830207 [DOI] [PubMed] [Google Scholar]
- Alvarez, W. (1922). The electrogastrogram and what it shows. JAMA, 78, 1116–1118. [Google Scholar]
- Amaris, M. A. , Sanmiguel, C. P. , Sadowski, D. C. , Bowes, K. L. , & Mintchev, M. P. (2002). Electrical activity from colon overlaps with normal gastric electrical activity in cutaneous recordings. Digestive Diseases and Sciences, 47(11), 6 10.1023/a:1020503908304 [DOI] [PubMed] [Google Scholar]
- Amassian, V. E. (1951). Cortical representation of visceral afferents. Journal of Neurophysiology, 14(6), 433–444. 10.1152/jn.1951.14.6.433 [DOI] [PubMed] [Google Scholar]
- Américo, M. F. , Miranda, J. R. A. , Corá, L. A. , & Romeiro, F. G. (2009). Electrical and mechanical effects of hyoscine butylbromide on the human stomach : A non‐invasive approach. Physiological Measurement, 30(4), 363–370. 10.1088/0967-3334/30/4/002 [DOI] [PubMed] [Google Scholar]
- Andreis, U. , Américo, M. F. , Corá, L. A. , Oliveira, R. B. , Baffa, O. , & Miranda, J. R. A. (2008). Gastric motility evaluated by electrogastrography and alternating current biosusceptometry in dogs. Physiological Measurement, 29(9), 1023–1031. 10.1088/0967-3334/29/9/002 [DOI] [PubMed] [Google Scholar]
- Andrews, P. L. , & Scratcherd, T. (1980). The gastric motility patterns induced by direct and reflex excitation of the vagus nerves in the anaesthetized ferret. The Journal of Physiology, 302(1), 363–378. 10.1113/jphysiol.1980.sp013248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli, T. R. , Cheng, L. K. , Du, P. , Wang, T.‐H. , Bernard, C. E. , Vannucchi, M.‐G. , … O’Grady, G. (2015). Loss of Interstitial Cells of Cajal and patterns of gastric dysrhythmia in patients with chronic unexplained nausea and vomiting. Gastroenterology, 149(1), 56–66.e5. 10.1053/j.gastro.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli, T. R. , Du, P. , Paskaranandavadivel, N. , Janssen, P. W. M. , Beyder, A. , Lentle, R. G. , … O’Grady, G. (2013). The bioelectrical basis and validity of gastrointestinal extracellular slow wave recordings : Bioelectrical basis of GI extracellular recordings. The Journal of Physiology, 591(18), 4567–4579. 10.1113/jphysiol.2013.254292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz, F. , & Malagelada, J.‐R. (1990). Perception and reflex relaxation of the stomach in response to gut distention. Gastroenterology, 98(5), 1193–1198. 10.1016/0016-5085(90)90333-V [DOI] [PubMed] [Google Scholar]
- Azzalini, D. , Rebollo, I. , & Tallon‐Baudry, C. (2019). Visceral signals shape brain dynamics and cognition. Trends in Cognitive Sciences, 23(6), 488–509. 10.1016/j.tics.2019.03.007 [DOI] [PubMed] [Google Scholar]
- Baldaro, B. , Battacchi, M. W. , Codispoti, M. , Tuozzi, G. , Trombini, G. , Bolzani, R. , & Palomba, D. (1996). Modifications of electrogastrographic activity during the viewing of brief film sequences. Perceptual and Motor Skills, 82(3_suppl), 1243–1250. 10.2466/pms.1996.82.3c.1243 [DOI] [PubMed] [Google Scholar]
- Baldaro, B. , Battacchi, M. W. , Trombini, G. , Palomba, D. , & Stegagno, L. (1990). Effects of an emotional negative stimulus on the cardiac, electrogastrographic, and respiratory responses. Perceptual and Motor Skills, 71(2), 647–655. 10.2466/pms.1990.71.2.647 [DOI] [PubMed] [Google Scholar]
- Baldaro, B. , Mazzetti, M. , Codispoti, M. , Tuozzi, G. , Bolzani, R. , & Trombini, G. (2001). Autonomic reactivity during viewing of an unpleasant film. Perceptual and Motor Skills, 93(3), 797–805. 10.2466/pms.2001.93.3.797 [DOI] [PubMed] [Google Scholar]
- Bayguinov, O. , Hennig, G. W. , & Sanders, K. M. (2011). Movement based artifacts may contaminate extracellular electrical recordings from GI muscles: Movement artifacts in extracellular recording. Neurogastroenterology & Motility, 23(11), 1029–e498. 10.1111/j.1365-2982.2011.01784.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud, H. R. , Blackshaw, L. A. , Brookes, S. J. H. , & Grundy, D. (2004). Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterology and Motility, 16(Suppl 1), 28–33. 10.1111/j.1743-3150.2004.00471.x [DOI] [PubMed] [Google Scholar]
- Bester, H. , Besson, J. M. , & Bernard, J. F. (1997). Organization of efferent projections from the parabrachial area to the hypothalamus : A Phaseolus vulgaris‐leucoagglutinin study in the rat. The Journal of Comparative Neurology, 383(3), 245–281. [DOI] [PubMed] [Google Scholar]
- Birmingham, A. (1898). The arrangement of the muscular fibres of the stomach. Transactions of the Royal Academy of Medicine in Ireland, 16(1), 432–440. 10.1007/BF03177287 [DOI] [Google Scholar]
- Bishop, V. S. , Malliani, A. , & Thorén, P. (1983). Handbook of physiology, section 2: The cardiovascular system In Shepard J. T. (Ed.), Comprehensive physiology (pp. 497–555). Baltimore: Waverly Press; 10.1002/cphy.cp020315 [DOI] [Google Scholar]
- Blackshaw, L. A. , Brookes, S. J. H. , Grundy, D. , & Schemann, M. (2007). Sensory transmission in the gastrointestinal tract. Neurogastroenterology and Motility, 19(1 Suppl), 1–19. 10.1111/j.1365-2982.2006.00871.x [DOI] [PubMed] [Google Scholar]
- Bozler, E. (1945). The action potentials of the stomach. American Journal of Physiology, 144, 693–700. 10.1152/ajplegacy.1945.144.5.693 [DOI] [PubMed] [Google Scholar]
- Bradshaw, L. A. , Cheng, L. K. , Chung, E. , Obioha, C. B. , Erickson, J. C. , Gorman, B. L. , … Richards, W. O. (2016). Diabetic gastroparesis alters the biomagnetic signature of the gastric slow wave. Neurogastroenterology and Motility, 28(6), 837–848. 10.1111/nmo.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B. H. , Smallwood, R. H. , Duthie, H. L. , & Stoddard, C. J. (1975). Intestinal smooth muscle electrical potentials recorded from surface electrodes. Medical & Biological Engineering, 13(1), 97–103. 10.1007/BF02478194 [DOI] [PubMed] [Google Scholar]
- Bruinstroop, E. , Cano, G. , VanderHorst, V. G. J. M. , Cavalcante, J. C. , Wirth, J. , Sena‐Esteves, M. , & Saper, C. B. (2012). Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. The Journal of Comparative Neurology, 520(9) , 1985–2001. 10.1002/cne.23024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, W. B. (1927). The James‐Lange theory of emotions : A critical examination and an alternative theory. The American Journal of Psychology, 39, 106–124. 10.2307/1415404 [DOI] [PubMed] [Google Scholar]
- Cechetto, D. F. , & Saper, C. B. (1987). Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. The Journal of Comparative Neurology, 262(1), 27–45. 10.1002/cne.902620104 [DOI] [PubMed] [Google Scholar]
- Chang, F.‐Y. (2005). Electrogastrography: Basic knowledge, recording, processing and its clinical applications. Journal of Gastroenterology and Hepatology, 20, 502–516. 10.1111/j.1440-1746.2004.03751.x [DOI] [PubMed] [Google Scholar]
- Chang, H. Y. , Mashimo, H. , & Goyal, R. K. (2003). IV. Current concepts of vagal efferent projections to the gut. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 284(3), G357–G366. 10.1152/ajpgi.00478.2002 [DOI] [PubMed] [Google Scholar]
- Chen, D. D. , Xu, X. , Wang, Z. , & Chen, J. D. Z. (2005). Alteration of gastric myoelectrical and autonomic activities with audio stimulation in healthy humans. Scandinavian Journal of Gastroenterology, 40, 814–821. 10.1080/00365520510015656 [DOI] [PubMed] [Google Scholar]
- Chen, D. D. , Xu, X. , Zhao, Q. , Yin, J. , Sallam, H. , & Chen, J. D. (2008). Effects of audio stimulation on gastric myoelectrical activity and sympathovagal balance in healthy adolescents and adults. Journal of Gastroenterology and Hepatology, 23, 141–149. 10.1111/j.1440-1746.2007.05123.x [DOI] [PubMed] [Google Scholar]
- Chen, J. , & McCallum, R. W. (1992). Gastric slow wave abnormalities in patients with gastroparesis. American Journal of Gastroenterology, 87, 477–482. [PubMed] [Google Scholar]
- Chen, J. D. , Schirmer, B. D. , & McCallum, R. W. (1994). Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 266(1), G90–G98. 10.1152/ajpgi.1994.266.1.G90 [DOI] [PubMed] [Google Scholar]
- Chen, J. D. Z. , Zou, X. , Lin, X. , Ouyang, S. , & Liang, J. (1999). Detection of gastric slow wave propagation from the cutaneous electrogastrogram. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 277(2), G424–G430. 10.1152/ajpgi.1999.277.2.G424 [DOI] [PubMed] [Google Scholar]
- Cheng, L. K. , Du, P. , & O’Grady, G. (2013). Mapping and modeling gastrointestinal bioelectricity : From Engineering Bench to Bedside. Physiology, 28(5), 310–317. 10.1152/physiol.00022.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba, T. , Kudara, N. , Sato, M. , Inomata, M. , Orii, S. , & Suzuki, K. (2007). Effect of a muscarinic M3 receptor agonist on gastric motility. Journal of Gastroenterology and Hepatology, 22(11), 2039–2041. 10.1111/j.1440-1746.2006.03363.x [DOI] [PubMed] [Google Scholar]
- Christensen, J. , Schedl, H. P. , & Clifton, J. A. (1966). The small intestinal basic electrical rhythm (slow wave) frequency gradient in normal men and in patients with a variety of diseases. Gastroenterology, 50(3), 309–315. 10.1016/S0016-5085(66)80069-0 [DOI] [PubMed] [Google Scholar]
- Christensen, J. , & Torres, E. I. (1975). Three layers of the opossum stomach : Responses to nerve stimulation. Gastroenterology, 69(3), 641–648. 10.1016/S0016-5085(19)32457-6 [DOI] [PubMed] [Google Scholar]
- Christoff, K. , Cosmelli, D. , Legrand, D. , & Thompson, E. (2011). Specifying the self for cognitive neuroscience. Trends in Cognitive Sciences, 15(3), 104–112. 10.1016/j.tics.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Coen, S. J. , Hobson, A. R. , & Aziz, Q. (2012). Chapter 23—Processing of gastrointestinal sensory signals in the brain In Johnson L. R., Ghishan F. K., Kaunitz J. D., Merchant J. L., Said H. M., & Wood J. D. (Eds.), Physiology of the gastrointestinal tract (5th ed., pp. 689–702). Boston: Academic Press; ). 10.1016/B978-0-12-382026-6.00023-3s [DOI] [Google Scholar]
- Cohen, M. X. (2014). Analyzing neural time series data: Theory and practice. Cambridge, MA: The MIT Press. [Google Scholar]
- Coizet, V. , Dommett, E. J. , Klop, E. M. , Redgrave, P. , & Overton, P. G. (2010). The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience, 168(1), 263–272. 10.1016/j.neuroscience.2010.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleski, R. , & Hasler, W. L. (2004). Directed endoscopic mucosal mapping of normal and dysrhythmic gastric slow waves in healthy humans. Neurogastroenterology and Motility, 16(5), 557–565. 10.1111/j.1365-2982.2004.00542.x [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , & Harrison, N. A. (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Cucchiara, S. , Salvia, G. , Borrelli, O. , Ciccimarra, E. , Az‐Zeqeh, N. , Rapagiolo, S. , … Riezzo, G. (1997). Gastric electrical dysrhythmias and delayed gastric emptying in gastroesophageal reflux disease. The American Journal of Gastroenterology, 92(7), 1103–1108. [PubMed] [Google Scholar]
- Damasio, A. R. (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 351(1346), 1413–1420. 10.1098/rstb.1996.0125 [DOI] [PubMed] [Google Scholar]
- Davis, R. C. , & Berry, F. (1963). Gastrointestinal reactions during a noise avoidance task. Psychological Reports, 12(1), 135–137. 10.2466/pr0.1963.12.1.135 [DOI] [Google Scholar]
- Davis, R. C. , Berry, F. , & Paden, A. (1969). The effect of certain tasks and conditions on gastrointestinal activity (Technical Report). Bloomington, IN: Indiana University. [Google Scholar]
- Davis, R. C. , Garafolo, L. , & Kveim, K. (1959). Conditions associated with gastrointestinal activity. Journal of Comparative and Physiological Psychology, 52(4), 466–475. 10.1037/h0044130 [DOI] [PubMed] [Google Scholar]
- Deuchars, S. A. , & Lall, V. K. (2015). Sympathetic preganglionic neurons: Properties and inputs. Comprehensive Physiology, 5, 829–869. [DOI] [PubMed] [Google Scholar]
- Downman, C. B. B. (1951). Cerebral destination of splanchnic afferent impulses. The Journal of Physiology, 113(4), 434–441. 10.1113/jphysiol.1951.sp004586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, P. , O’Grady, G. , Cheng, L. K. , & Pullan, A. J. (2010). A multiscale model of the electrophysiological basis of the human electrogastrogram. Biophysical Journal, 99(9), 2784–2792. 10.1016/j.bpj.2010.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum, R. P. , Levinthal, D. J. , & Strick, P. L. (2009). The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. The Journal of Neuroscience, 29(45), 14223–14235. 10.1523/JNEUROSCI.3398-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolani, M. , Baldaro, B. , Comani, G. , De Portu, I. , Rossi, N. , & Trombini, G. (1982). Evolution de la motilité gastrique, de la fréquence cardiaque et ventilatoire de sujets sains pendant L’exécution d’épreuves de performance. Aggressologie, 23, 263–267. [PubMed] [Google Scholar]
- Ercolani, M. , Baldaro, B. , & Trombini, G. (1989). Effects of two tasks and two levels of difficulty upon surface electrogastrograms. Perceptual and Motor Skills, 69(1), 99–110. 10.2466/pms.1989.69.1.99 [DOI] [PubMed] [Google Scholar]
- Erickson, J. C. , Bruce, L. E. , Taylor, A. , Richman, J. , Higgins, C. , Wells, C. I. , & O’Grady, G. (2019). Electrocolonography : Non‐invasive detection of colonic cyclic motor activity from multielectrode body surface recordings. IEEE Transactions on Bio‐Medical Engineering, 10.1109/TBME.2019.2941851 [DOI] [PubMed] [Google Scholar]
- Erişir, A. , Van Horn, S. C. , & Sherman, S. M. (1997). Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proceedings of the National Academy of Sciences of the United States of America, 94(4), 1517–1520. 10.1073/pnas.94.4.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familoni, B. O. , Kingma, Y. J. , & Bowes, K. L. (1987). Study of transcutaneous and intraluminal measurement of gastric electrical activity in humans. Medical & Biological Engineering & Computing, 25(4), 397–402. 10.1007/BF02443360 [DOI] [PubMed] [Google Scholar]
- Fedor, J. H. , & Russell, R. W. (1965). Gastrointestinal reactions to response‐contingent stimulation. Psychological Reports, 16(1), 95–113. 10.2466/pr0.1965.16.1.95 [DOI] [PubMed] [Google Scholar]
- Foley, J. O. (1948). The functional types of nerve fibers and their numbers in the great splanchnic nerve. The Anatomical Record, 100, 766–767. [Google Scholar]
- Fritsch, H. , & Kühnel, W. (2008). Color atlas of human anatomy: Internal organs (Vol. 2). Stuttgart, Germany: Georg Thieme Verlag. [Google Scholar]
- Fulwiler, C. E. , & Saper, C. B. (1984). Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research Reviews, 7(3), 229–259. 10.1016/0165-0173(84)90012-2 [DOI] [PubMed] [Google Scholar]
- Furness, J. B. (2006). The enteric nervous system. Oxford, UK: Blackwell Publishing. [Google Scholar]
- Furness, J. B. (2012). The enteric nervous system and neurogastroenterology. Nature Reviews Gastroenterology & Hepatology, 9(5), 286–294. 10.1038/nrgastro.2012.32 [DOI] [PubMed] [Google Scholar]
- Geldof, H. , van der Schee, E. J. , Smout, A. J. P. M. , van de Merwe, J. P. , van Blankenstein, M. , & Grashuis, J. L. (1989). Myoelectrical activity of the stomach in gastric ulcer patients: An electrogastrographic study. Neurogastroenterology & Motility, 1(2), 122–130. 10.1111/j.1365-2982.1989.tb00150.x [DOI] [Google Scholar]
- Gharibans, A. A. , Coleman, T. P. , Mousa, H. , & Kunkel, D. C. (2019). Spatial patterns from high‐resolution electrogastrography correlate with severity of symptoms in patients with functional dyspepsia and gastroparesis. Clinical Gastroenterology and Hepatology, 17(13), 2668–2677. 10.1016/j.cgh.2019.04.039 [DOI] [PubMed] [Google Scholar]
- Gharibans, A. A. , Kim, S. , Kunkel, D. C. , & Coleman, T. P. (2017). High‐resolution Electrogastrogram: A novel, noninvasive method for determining gastric slow‐wave direction and speed. IEEE Transactions on Biomedical Engineering, 64(4), 807–815. 10.1109/TBME.2016.2579310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibans, A. A. , Smarr, B. L. , Kunkel, D. C. , Kriegsfeld, L. J. , Mousa, H. M. , & Coleman, T. P. (2018). Artifact rejection methodology enables continuous, noninvasive measurement of gastric myoelectric activity in ambulatory subjects. Scientific Reports, 8(1), 1–12. 10.1038/s41598-018-23302-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros, P. J. , Quigley, K. S. , & Mordkoff, J. T. (2001). Gastric myoelectrical and autonomic cardiac reactivity to laboratory stressors. Psychophysiology, 38(4), 540–547. [PMC free article] [PubMed] [Google Scholar]
- Gillis, R. A. , Quest, J. A. , Pagani, F. D. , & Norman, W. P. (1989). Control centers in the central nervous system for regulating gastrointestinal motility In Wood J. D. (Ed.), Handbook of physiology. The gastrointestinal system. Motility and circulation (Vol. 1, pp. 621–683). Bethesda, MD: American Physiological Society; 10.1002/cphy.cp060117 [DOI] [Google Scholar]
- Hall, K. E. , el‐Sharkawy, T. Y. , & Diamant, N. E. (1986). Vagal control of canine postprandial upper gastrointestinal motility. The American Journal of Physiology, 250(4 Pt 1), G501–G510. 10.1152/ajpgi.1986.250.4.G501 [DOI] [PubMed] [Google Scholar]
- Hamilton, J. W. , Bellahsene, B. E. , Reichelderfer, M. , Webster, J. G. , & Bass, P. (1986). Human electrogastrograms: Comparison of surface and mucosal recordings. Digestive Diseases and Sciences, 31(1), 33–39. 10.1007/BF01347907 [DOI] [PubMed] [Google Scholar]
- Han, W. , Tellez, L. A. , Perkins, M. H. , Perez, I. O. , Qu, T. , Ferreira, J. , … de Araujo, I. E. (2018). A neural circuit for gut‐induced reward. Cell, 175(3), 665–678.e23. 10.1016/j.cell.2018.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, N. A. , Gray, M. A. , Gianaros, P. J. , & Critchley, H. D. (2010). The embodiment of emotional feelings in the brain. Journal of Neuroscience, 30(38), 12878–12884. 10.1523/JNEUROSCI.1725-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder, R. A. , & Kelly, K. A. (1977). Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. The American Journal of Surgery, 133, 29–33. 10.1016/0002-9610(77)90187-8 [DOI] [PubMed] [Google Scholar]
- Hirst, G. D. S. , & Edwards, F. R. (2006). Electrical events underlying organized myogenic contractions of the guinea pig stomach: Descending propagation of gastric slow waves. The Journal of Physiology, 576, 659–665. 10.1113/jphysiol.2006.116491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocke, M. , Schöne, U. , Richert, H. , Görnert, P. , Keller, J. , Layer, P. , & Stallmach, A. (2009). Every slow‐wave impulse is associated with motor activity of the human stomach. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 296(4), G709–G716. 10.1152/ajpgi.90318.2008 [DOI] [PubMed] [Google Scholar]
- Holzer, P. (2006). Efferent‐like roles of afferent neurons in the gut: Blood flow regulation and tissue protection. Autonomic Neuroscience: Basic & Clinical, 125, 70–75. 10.1016/j.autneu.2006.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzl, R. , Schroder, G. , & Kiefer, L. (1979). Indirect gastrointestinal motility measurement for use in experimental psychosomatics: A new method and some data. Behavior Analysis and Modification, 3, 77–97. [Google Scholar]
- Homma, S. , Hasegawa, J. , Maruta, T. , Watanabe, N. , Matsuo, H. , Tamiya, Y. , … Hatakeyama, K. (1999). Isopower maps of the electrogastrogram (EGG) after total gastrectomy or total colectomy. Neurogastroenterology & Motility, 11, 441–448. 10.1046/j.1365-2982.1999.00170.x [DOI] [PubMed] [Google Scholar]
- Homma, S. , Shimakage, N. , Yagi, M. , Hasegawa, J. , Sato, K. , Matsuo, H. , … Hatakeyama, K. (1995). Electrogastrography prior to and following total gastrectomy, subtotal gastrectomy, and gastric tube formation. Digestive Diseases and Sciences, 40(4), 893–900. 10.1007/BF02064997 [DOI] [PubMed] [Google Scholar]
- Hou, X. , Yin, J. , Liu, J. , Pasricha, P. J. , & Chen, J. D. Z. (2005). In vivo gastric and intestinal slow waves in W/Wv Mice. Digestive Diseases and Sciences, 50(7), 1335–1341. 10.1007/s10620-005-2783-6 [DOI] [PubMed] [Google Scholar]
- Huizinga, J. D. , & Chen, J.‐H. (2014). Interstitial Cells of Cajal: Update on basic and clinical science. Current Gastroenterology Reports, 16(1), 363 10.1007/s11894-013-0363-z [DOI] [PubMed] [Google Scholar]
- Hylden, J. L. , Hayashi, H. , Bennett, G. J. , & Dubner, R. (1985). Spinal lamina I neurons projecting to the parabrachial area of the cat midbrain. Brain Research, 336(1), 195–198. 10.1016/0006-8993(85)90436-6 [DOI] [PubMed] [Google Scholar]
- Imai, K. , & Sakita, M. (2005). Pre‐ and postoperative electrogastrography in patients with gastric cancer. Hepato‐Gastroenterology, 52(62), 639–644. [PubMed] [Google Scholar]
- Indrio, F. , Riezzo, G. , Raimondi, F. , Bisceglia, M. , Cavallo, L. , & Francavilla, R. (2009). Effects of probiotic and prebiotic on gastrointestinal motility in newborns. Journal of Physiology and Pharmacology, 60(Suppl 6), 27–31. [PubMed] [Google Scholar]
- James, W. (1890). The Principles of Psychology. New York: Holt. [Google Scholar]
- Jonderko, K. , Kasicka‐Jonderko, A. , & Blonska‐Fajfrowska, B. (2005). Does body posture affect the parameters of a cutaneous electrogastrogram? Journal of Smooth Muscle Research, 41(3), 133–140. 10.1540/jsmr.41.133 [DOI] [PubMed] [Google Scholar]
- Kaiho, T. , Shimoyama, I. , Nakajima, Y. , & Ochiai, T. (2000). Gastric and non‐gastric signals in electrogastrography. Journal of the Autonomic Nervous System, 79(1), 60–66. 10.1016/S0165-1838(99)00098-3 [DOI] [PubMed] [Google Scholar]
- Kaneoke, Y. , Koike, Y. , Sakurai, N. , Washimi, Y. , Hirayama, M. , Hoshiyama, M. , & Takahashi, A. (1995). Gastrointestinal dysfunction in Parkinson’s disease detected by electrogastroenterography. Journal of the Autonomic Nervous System, 50(3), 275–281. 10.1016/0165-1838(94)00098-5 [DOI] [PubMed] [Google Scholar]
- Kelly, K. A. (1980). Gastric emptying of liquids and solids : Roles of proximal and distal stomach. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 239(2), G71–G76. 10.1152/ajpgi.1980.239.2.G71 [DOI] [PubMed] [Google Scholar]
- Kelly, K. A. , Code, C. F. , & Elveback, L. R. (1969). Patterns of canine gastric electrical activity. American Journal of Physiology, 217, 461–470. 10.1152/ajplegacy.1969.217.2.461 [DOI] [PubMed] [Google Scholar]
- Kern, M. , Aertsen, A. , Schulze‐Bonhage, A. , & Ball, T. (2013). Heart cycle‐related effects on event‐related potentials, spectral power changes, and connectivity patterns in the human ECoG. NeuroImage, 81, 178–190. 10.1016/j.neuroimage.2013.05.042 [DOI] [PubMed] [Google Scholar]
- Khalsa, S. S. , Adolphs, R. , Cameron, O. G. , Critchley, H. D. , Davenport, P. W. , Feinstein, J. S. , … Kupli, R. (2018). Interoception and mental health: A roadmap. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 3(6), 501–513. 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüppel, M. , Huizinga, J. D. , Malysz, J. , & Bernstein, A. (1998). Developmental origin and kit‐dependent development of the Interstitial Cells of Cajal in the mammalian small intestine. Developmental Dynamics, 211(1), 60–71. https://10.1002/(SICI)1097‐0177(199801)211:1<60:AID‐AJA6>3.0.CO;2‐5 [DOI] [PubMed] [Google Scholar]
- Koch, K. L. , Bingaman, S. , Tan, L. , & Stern, R. M. (1998). Visceral perceptions and gastric myoelectrical activity in healthy women and in patients with bulimia nervosa. Neurogastroenterology & Motility, 10, 3–10. 10.1046/j.1365-2982.1998.00080.x [DOI] [PubMed] [Google Scholar]
- Koch, K. L. , Hong, S. P. , & Xu, L. (2000). Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility‐like dyspepsia symptoms and in control subjects. Journal of Clinical Gastroenterology, 31, 125–129. 10.1097/00004836-200009000-00007 [DOI] [PubMed] [Google Scholar]
- Koch, K. L. , & Stern, R. M. (2004). Handbook of electrogastrography. New York, NY: Oxford University Press. [Google Scholar]
- Květina, J. , Varayil, J. , Ali, S. , Kuneš, M. , Bureš, J. , Tachecí, I. , … Kopáčová, M. (2010). Preclinical electrogastrography in experimental pigs. Interdisciplinary Toxicology, 3(2), 53–58. 10.2478/v10102-010-0011-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladabaum, U. , Minoshima, S. , Hasler, W. L. , Cross, D. , Chey, W. D. , & Owyang, C. (2001). Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology, 120(2), 369–376. 10.1053/gast.2001.21201 [DOI] [PubMed] [Google Scholar]
- Lange, C. G. (1885). The Emotions (William & Wilkins). Baltimore, MD: Williams & Wilkins. [Google Scholar]
- Leek, B. F. (1972).Abdominal visceral receptors In Andersson B., Fillenz M., Hellon R. F., Howe A., Leek B. F., Neil E. (Eds.), Enteroceptors (pp. 113–160). Berlin, Heidelberg: Springer; 10.1007/978-3-642-65252-3_4 [DOI] [Google Scholar]
- Liang, J. , & Chen, J. D. (1997). What can be measured from surface electrogastrography. Computer Simulationss Digestive Diseases and Sciences, 42(7), 1331–1343. 10.1023/a:1018869300296 [DOI] [PubMed] [Google Scholar]
- Lin, H.‐H. , Chang, W.‐K. , Chu, H.‐C. , Huang, T.‐Y. , Chao, Y.‐C. , & Hsieh, T.‐Y. (2007). Effects of music on gastric myoelectrical activity in healthy humans. International Journal of Clinical Practice, 61(7), 1126–1130. 10.1111/j.1742-1241.2006.01090.x [DOI] [PubMed] [Google Scholar]
- Lin, Z. , Chen, J. D. Z. , Schirmer, B. D. , & McCallum, R. W. (2000). Postprandial response of gastric slow waves : Correlation of serosal recordings with the electrogastrogram. Digestive Diseases and Sciences, 45(4), 7 10.1023/a:1005434020310 [DOI] [PubMed] [Google Scholar]
- Lin, Z. , Eaker, E. Y. , Sarosiek, I. , & McCallum, R. W. (1999). Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. American Journal of Gastroenterology, 94(9), 2384–2389. 10.1111/j.1572-0241.1999.01362.x [DOI] [PubMed] [Google Scholar]
- Lindberg, G. , Iwarzon, M. , & Hammarlund, B. (1996). 24‐hour ambulatory electrogastrography in healthy volunteers. Scandinavian Journal of Gastroenterology, 31(7), 658–664. 10.3109/00365529609009146 [DOI] [PubMed] [Google Scholar]
- Linsong, W. , Huailin, Z. , Xitai, L. , Xiaojin, Z. , & Pingan, R. (1989). The primary research of electrogastrograms in macaca mulatta of taihang mountains. Journal of Henan Normal University (Natural Science). [Google Scholar]
- Lu, C.‐L. , Wu, Y.‐T. , Yeh, T.‐C. , Chen, L.‐F. , Chang, F.‐Y. , Lee, S.‐D. , … Hsieh, J.‐C. (2004). Neuronal correlates of gastric pain induced by fundus distension: A 3T‐fMRI study. Neurogastroenterology and Motility, 16(5), 575–587. 10.1111/j.1365-2982.2004.00562.x [DOI] [PubMed] [Google Scholar]
- Lu, S. M. , Guido, W. , & Sherman, S. M. (1993). The brain‐stem parabrachial region controls mode of response to visual stimulation of neurons in the cat’s lateral geniculate nucleus. Visual Neuroscience, 10(4), 631–642. 10.1017/s0952523800005332 [DOI] [PubMed] [Google Scholar]
- Martin, A. , Nicolov, N. , Ormieres, D. , Beloncle, M. , & Murat, J. (1982). La motricité gastrique chez 60 sujets normaux sous l’influence d’une tension émotionnelle, premiers résultats. Aggressologie, 23, 269–273. [PubMed] [Google Scholar]
- McCallum, R. , Jones, T. , Lin, Z. , Sarosiek, I. , & Moncure, M. (2001). Assessment of gastric emptying and myoelectric activity in the morbidly obese patient. American Gastroenterological Association, 120(5), A290. [Google Scholar]
- Meissner, K. , Muth, E. R. , & Herbert, B. M. (2011). Bradygastric activity of the stomach predicts disgust sensitivity and perceived disgust intensity. Biological Psychology, 86(1), 9–16. 10.1016/j.biopsycho.2010.09.014 [DOI] [PubMed] [Google Scholar]
- Mintchev, M. P. , Kingma, Y. J. , & Bowes, K. L. (1993). Accuracy of cutaneous recordings of gastric electrical activity. Gastroenterology, 104(5), 1273–1280. 10.1016/0016-5085(93)90334-9 [DOI] [PubMed] [Google Scholar]
- Mintchev, M. , Otto, S. , & Bowes, K. (1997). Electrogastrography can recognize gastric electrical uncoupling in dogs. Gastroenterology, 112(6), 2006–2011. 10.1053/gast.1997.v112.pm9178693 [DOI] [PubMed] [Google Scholar]
- Morrison, S. F. , Sved, A. F. , & Passerin, A. M. (1999). GABA‐mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 276(2), R290–R297. 10.1152/ajpregu.1999.276.2.R290 [DOI] [PubMed] [Google Scholar]
- Munk, M. H. , Roelfsema, P. R. , König, P. , Engel, A. K. , & Singer, W. (1996). Role of reticular activation in the modulation of intracortical synchronization. Science, 272, 271–274. 10.1126/science.272.5259.271 [DOI] [PubMed] [Google Scholar]
- Murakami, H. , Matsumoto, H. , Ueno, D. , Kawai, A. , Ensako, T. , Kaida, Y. , … Hirai, T. (2013). Current status of multichannel electrogastrography and examples of its use. Journal of Smooth Muscle Research, 49, 78–88. 10.1540/jsmr.49.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth, E. R. , Koch, K. L. , Stern, R. M. , & Thayer, J. F. (1999). Effect of autonomic nervous system manipulations on gastric myoelectrical activity and emotional responses in healthy human subjects. Psychosomatic Medicine, 61(3), 297–303. 10.1097/00006842-199905000-00008 [DOI] [PubMed] [Google Scholar]
- Norgren, R. (1978). Projections from the nucleus of the solitary tract in the rat. Neuroscience, 3(2), 207–218. 10.1016/0306-4522(78)90102-1 [DOI] [PubMed] [Google Scholar]
- O’Grady, G. (2012). Gastrointestinal extracellular electrical recordings: Fact or artifact? Neurogastroenterology and Motility, 24(1), 1–6. 10.1111/j.1365-2982.2011.01815.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady, G. , Du, P. , Cheng, L. K. , Egbuji, J. U. , Lammers, W. J. E. P. , Windsor, J. A. , & Pullan, A. J. (2010). Origin and propagation of human gastric slow‐wave activity defined by high‐resolution mapping. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 299(3), G585–G592. 10.1152/ajpgi.00125.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady, G. , Angeli, T. R. , Du, P. , Lahr, C. , Lammers, W. J. E. P. , Windsor, J. A. , … Cheng, L. K. (2012). Abnormal initiation and conduction of slow‐wave activity in gastroparesis, defined by high‐resolution electrical mapping. Gastroenterology, 143(3), 589–598.e3. 10.1053/j.gastro.2012.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obioha, C. , Erickson, J. , Suseela, S. , Hajri, T. , Chung, E. , … Bradshaw, L. A. (2016). Effect of Body Mass Index on the sensitivity of magnetogastrogram and electrogastrogram. Journal of Gastroenterology and Hepatology Research, 2(4), 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 1–9. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani, F. D. , Norman, W. P. , Kasbekar, D. K. , & Gillis, R. A. (1985). Localization of sites within dorsal motor nucleus of vagus that affect gastric motility. The American Journal of Physiology, 249(1 Pt 1), G73–G84. 10.1152/ajpgi.1985.249.1.G73 [DOI] [PubMed] [Google Scholar]
- Parkman, H. P. , Harris, A. D. , Miller, M. A. , & Fisher, R. S. (1996). Influence of age, gender, and menstrual cycle on the normal electrogastrogram. The American Journal of Gastroenterology, 91(1), 127–133. [PubMed] [Google Scholar]
- Parkman, H. P. , Hasler, W. L. , Barnett, J. L. , & Eaker, E. Y. (2003). Electrogastrography : A document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterology and Motility, 15(2), 89–102. 10.1046/j.1365-2982.2003.00396.x [DOI] [PubMed] [Google Scholar]
- Parkman, H. P. , & Orr, W. C. (2007). The gastrointestinal motility laboratory. Gastroenterology Clinics of North America, 36(3), 515–529. 10.1016/j.gtc.2007.07.010 [DOI] [PubMed] [Google Scholar]
- Paton, J. F. , & Kasparov, S. (2000). Sensory channel specific modulation in the nucleus of the solitary tract. Journal of the Autonomic Nervous System, 80(3), 117–129. 10.1016/s0165-1838(00)00077-1 [DOI] [PubMed] [Google Scholar]
- Peupelmann, J. , Quick, C. , Berger, S. , Hocke, M. , Tancer, M. E. , Yeragani, V. K. , & Bär, K.‐J. (2009). Linear and non‐linear measures indicate gastric dysmotility in patients suffering from acute schizophrenia. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 33(7), 1236–1240. 10.1016/j.pnpbp.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Pezzolla, F. , Riezzo, G. , Maselli, M. A. , & Giorgio, I. (1989). Electrical activity recorded from abdominal surface after gastrectomy or colectomy in humans. Gastroenterology, 97(2), 313–320. 10.1016/0016-5085(89)90066-8 [DOI] [PubMed] [Google Scholar]
- Pfaffenbach, B. , Adamek, R. J. , Kuhn, K. , & Wegener, M. (1995). Electrogastrography in healthy subjects: Evaluation of normal values, influence of age and gender. Digestive Diseases and Sciences, 40(7), 1445–1450. 10.1007/BF02285190 [DOI] [PubMed] [Google Scholar]
- Powley, T. L. , & Phillips, R. J. (2011). Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: Analogues of muscle spindle organs? Neuroscience, 186, 188–200. 10.1016/j.neuroscience.2011.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley, T. L. , Wang, X.‐Y. , Fox, E. A. , Phillips, R. J. , Liu, L. W. C. , & Huizinga, J. D. (2008). Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterology and Motility, 20(1), 69–79. 10.1111/j.1365-2982.2007.00990.x [DOI] [PubMed] [Google Scholar]
- Pritchard, T. C. , Hamilton, R. B. , & Norgren, R. (2000). Projections of the parabrachial nucleus in the old world monkey. Experimental Neurology, 165(1), 101–117. 10.1006/exnr.2000.7450 [DOI] [PubMed] [Google Scholar]
- Quadt, L. , Critchley, H. D. , & Garfinkel, S. N. (2018). The neurobiology of interoception in health and disease. Annals of the New York Academy of Sciences, 1428(1), 112–128. 10.1111/nyas.13915 [DOI] [PubMed] [Google Scholar]
- Rayner, C. K. , Hebbard, G. S. , & Horowitz, M. (2012). Chapter 35—Physiology of the antral pump and gastric emptying In Johnson L. R., Ghishan F. K., Kaunitz J. D., Merchant J. L., Said H. M., & Wood J. D. (Eds.), Physiology of the gastrointestinal tract (5th ed., pp. 959–976). Boston, MA: Academic Press; 10.1016/B978-0-12-382026-6.00035-X [DOI] [Google Scholar]
- Rebollo, I. , Devauchelle, A.‐D. , Béranger, B. , & Tallon‐Baudry, C. (2018). Stomach‐brain synchrony reveals a novel, delayed‐connectivity resting‐state network in humans. Elife, 7, e33321 10.7554/eLife.33321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, C. G. , Babo‐Rebelo, M. , Schwartz, D. , & Tallon‐Baudry, C. (2017). Phase‐amplitude coupling at the organism level: The amplitude of spontaneous alpha rhythm fluctuations varies with the phase of the infra‐slow gastric basal rhythm. NeuroImage, 146, 951–958. 10.1016/j.neuroimage.2016.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezzo, G. , Chiloiro, M. , & Guerra, V. (1998). Electrogastrography in healthy children: Evaluation of normal values, influence of age, gender, and obesity. Digestive Diseases and Sciences, 43(8), 1646–1651. 10.1023/a:1018894511181 [DOI] [PubMed] [Google Scholar]
- Riezzo, G. , Pezzolla, F. , & Giorgio, I. (1991). Effects of age and obesity on fasting gastric electrical activity in man: A cutaneous electrogastrographic study. Digestion, 50, 176–181. 10.1159/000200759 [DOI] [PubMed] [Google Scholar]
- Riezzo, G. , Pezzolla, F. , Maselli, M. A. , & Giorgio, I. (1994). Electrical activity recorded from abdominal surface before and after right hemicolectomy in man. Digestion, 55(3), 185–190. 10.1159/000201146 [DOI] [PubMed] [Google Scholar]
- Riezzo, G. , Porcelli, P. , Guerra, V. , & Giorgio, I. (1996). Effects of different psychophysiological stressors on the cutaneous electrogastrogram in healthy subjects. Archives of Physiology and Biochemistry, 104(3), 282–286. 10.1076/apab.104.3.282.12899 [DOI] [PubMed] [Google Scholar]
- Riezzo, G. , Russo, F. , & Indrio, F. (2013). Electrogastrography in adults and children: The strength, pitfalls, and clinical significance of the cutaneous recording of the gastric electrical activity. BioMed Research International, 2013, 1–14. 10.1155/2013/282757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhland, C. , Koschke, M. , Greiner, W. , Peupelmann, J. , Pietsch, U. , Hocke, M. , … Bär, K.‐J. (2008). Gastric dysmotility in patients with major depression. Journal of Affective Disorders, 110(1–2), 185–190. 10.1016/j.jad.2007.12.236 [DOI] [PubMed] [Google Scholar]
- Sanders, K. M. , Koh, S. D. , & Ward, S. M. (2006). Interstitial Cells of Cajal as pacemakers in the gastrointestinal tract. Annual Review of Physiology, 68, 307–343. 10.1146/annurev.physiol.68.040504.094718 [DOI] [PubMed] [Google Scholar]
- Sanders, K. M. , Ward, S. M. , & Koh, S. D. (2014). Interstitial Cells: Regulators of smooth muscle function. Physiological Reviews, 94(3), 859–907. 10.1152/physrev.00037.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper, C. B. (2002). The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience, 25(1), 433–469. 10.1146/annurev.neuro.25.032502.111311 [DOI] [PubMed] [Google Scholar]
- Saper, C. B. , & Loewy, A. D. (1980). Efferent connections of the parabrachial nucleus in the rat. Brain Research, 197(2), 291–317. 10.1016/0006-8993(80)91117-8 [DOI] [PubMed] [Google Scholar]
- Schemann, M. , & Grundy, D. (1992). Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. The American Journal of Physiology, 263(5 Pt 1), G709–718. 10.1152/ajpgi.1992.263.5.G709 [DOI] [PubMed] [Google Scholar]
- Shimamoto, C. , Hirata, I. , Hiraike, Y. , Takeuchi, N. , Nomura, T. , & Katsu, K. (2002). Evaluation of gastric motor activity in the elderly by electrogastrography and the 13C‐Acetate Breath Test. Gerontology, 48, 381–386. 10.1159/000065500 [DOI] [PubMed] [Google Scholar]
- Shipley, M. T. , & Sanders, M. S. (1982). Special senses are really special: Evidence for a reciprocal, bilateral pathway between insular cortex and nucleus parabrachialis. Brain Research Bulletin, 8(5), 493–501. 10.1016/0361-9230(82)90007-7 [DOI] [PubMed] [Google Scholar]
- Simonian, H. P. , Panganamamula, K. , Parkman, H. P. , Xu, X. , Chen, J. Z. , Lindberg, G. , … Buhl, H. (2004). Multichannel electrogastrography (EGG) in normal subjects: A multicenter study. Digestive Diseases and Sciences, 49(4), 594–601. 10.1023/B:DDAS.0000026304.83214.50 [DOI] [PubMed] [Google Scholar]
- Smirnov, V. M. , & Lychkova, A. E. (2003). Mechanism of synergism between sympathetic and parasympathetic autonomic nervous systems in the regulation of motility of the stomach and sphincter of Oddi. Bulletin of Experimental Biology and Medicine, 135(4), 327–329. 10.1023/a:1024692226837 [DOI] [PubMed] [Google Scholar]
- Smout, A. J. P. M. , Van Der Schee, E. J. , & Grashuis, J. L. (1980). What is measured in electrogastrography? Digestive Diseases and Sciences, 25, 179–187. 10.1007/bf01308136 [DOI] [PubMed] [Google Scholar]
- Somarajan, S. , Cassilly, S. , Obioha, C. , Richards, W. O. , & Bradshaw, L. A. (2014). Effects of body mass index on gastric slow wave: A magnetogastrographic study. Physiological Measurement, 35(2), 205–215. 10.1088/0967-3334/35/2/205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. , Gorsuch, R. , Lushene, R. , Vagg, P. , & Jacobs, G. (1983). Manual for the state‐trait anxiety inventory. Palo Alto, CA: Consulting Psychologists. [Google Scholar]
- Stern, R. M. (1966). A re‐examination of the effects of response‐contingent aversive tones on gastrointestinal activity. Psychophysiology, 2(3), 217–223. 10.1111/j.1469-8986.1966.tb02645.x [DOI] [PubMed] [Google Scholar]
- Stern, R. M. (1983). Responsiveness of the stomach to environmental events Psychophysiology of the gastrointestinal tract: Experimental and clinical applications (pp. 181–207). New York, NY: Plenum. [Google Scholar]
- Stern, R. M. , Crawford, H. E. , Stewart, W. R. , Vasey, M. W. , & Koch, K. L. (1989). Sham feeding. Cephalic‐vagal influences on gastric myoelectric activity. Digestive Diseases and Sciences, 34(4), 521–527. 10.1007/bf01536327 [DOI] [PubMed] [Google Scholar]
- Stern, R. M. , Jokerst, M. D. , Levine, M. E. , & Koch, K. L. (2001). The stomach’s response to unappetizing food: cephalic‐vagal effects on gastric myoelectric activity. Neurogastroenterology & Motility, 13(2), 151–154. 10.1046/j.1365-2982.2001.00250.x [DOI] [PubMed] [Google Scholar]
- Stern, R. M. , Koch, K. L. , Leibowitz, H. W. , Lindblad, I. M. , Shupert, C. L. , & Stewart, W. R. (1985). Tachygastria and motion sickness. Aviation, Space, and Environmental Medicine, 56(11), 1074–1077. [PubMed] [Google Scholar]
- Stern, R. M. , Koch, K. L. , Levine, M. E. , & Muth, E. R. (2007). Gastrointestinal response In Cacioppo J. T., Tassinary L. G., & Berntson G. (Eds.), Handbook of psychophysiology (pp. 211–230). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Stern, R. M. , Koch, K. L. , Stewart, W. R. , & Vasey, M. W. (1987). Electrogastrography: Current issues in validation and methodology. Psychophysiology, 24, 55–64. 10.1111/j.1469-8986.1987.tb01862.x [DOI] [PubMed] [Google Scholar]
- Stern, R. M. , Vasey, M. W. , Senqi, H. , & Koch, K. L. (1991). Effects of cold stress on gastric myoelectric activity. Neurogastroenterology & Motility, 3, 225–228. 10.1111/j.1365-2982.1991.tb00065.x [DOI] [Google Scholar]
- Suzuki, N. , Prosser, C. L. , & Dahms, V. (1986). Boundary cells between longitudinal and circular layers: Essential for electrical slow waves in cat intestine. The American Journal of Physiology, 250(3 Pt 1), G287–294. 10.1152/ajpgi.1986.250.3.G287 [DOI] [PubMed] [Google Scholar]
- Sveshnikov, D. S. , Smirnov, V. M. , Myasnikov, I. L. , & Kuchuk, A. V. (2012). Study of the nature of sympathetic trunk nerve fibers enhancing gastric motility. Bulletin of Experimental Biology and Medicine, 152(3), 283–285. 10.1007/s10517-012-1508-z [DOI] [PubMed] [Google Scholar]
- Tack, J. (2012). Chapter 34—Neurophysiologic mechanisms of gastric reservoir function In Johnson L. R., Ghishan F. K., Kaunitz J. D., Merchant J. L., Said H. M., & Wood J. D. (Eds.), Physiology of the gastrointestinal tract (Fifth Edition, pp. 951–957). Boston, MA: Academic Press; 10.1016/B978-0-12-382026-6.00034-8 [DOI] [Google Scholar]
- Taylor, I. , Duthie, H. L. , Smallwood, R. , & Linkens, D. (1975). Large bowel myoelectrical activity in man. Gut, 16(10), 808–814. 10.1136/gut.16.10.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, E. , & Varela, F. J. (2001). Radical embodiment: Neural dynamics and consciousness. Trends in Cognitive Sciences, 5(10), 418–425. 10.1016/s1364-6613(00)01750-2 [DOI] [PubMed] [Google Scholar]
- Tolj, N. (2007). The impact of age, sex, body mass index and menstrual cycle phase on gastric myoelectrical activity characteristics in a healthy Croatian population. Collegium Antropologicum, 31(4), 955–962. [PubMed] [Google Scholar]
- Tosetti, C. , Corinaldesi, R. , Stanghellini, V. , Pasquali, R. , Corbelli, C. , Zoccoli, G. , … Barbara, L. (1996). Gastric emptying of solids in morbid obesity. International Journal of Obesity and Related Metabolic Disorders, 20, 200–205. [PubMed] [Google Scholar]
- Travagli, R. A. , Hermann, G. E. , Browning, K. N. , & Rogers, R. C. (2006). Brainstem circuits regulating gastric function. Annual Review of Physiology, 68, 279–305. 10.1146/annurev.physiol.68.040504.094635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpeer, I. , & Blitsten, P. (1926). Registration of peristalsis by the Einthoven galvanometer. The American Journal of Diseases of Children, 454–455. [Google Scholar]
- Uhlrich, D. J. , Cucchiaro, J. B. , & Sherman, S. M. (1988). The projection of individual axons from the parabrachial region of the brain stem to the dorsal lateral geniculate nucleus in the cat. The Journal of Neuroscience, 8(12), 4565–4575. 10.1523/JNEUROSCI.08-12-04565.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlrich, D. J. , Tamamaki, N. , Murphy, P. C. , & Sherman, S. M. (1995). Effects of brain stem parabrachial activation on receptive field properties of cells in the cat’s lateral geniculate nucleus. Journal of Neurophysiology, 73(6), 2428–2447. 10.1152/jn.1995.73.6.2428 [DOI] [PubMed] [Google Scholar]
- Umans, B. D. , & Liberles, S. D. (2018). Neural sensing of organ volume. Trends in Neurosciences, 41(12), 911–924. 10.1016/j.tins.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck, Z. , Vögele, C. , Blechert, J. , Lutz, A. P. C. , Schulz, A. , & Herbert, B. M. (2016). The water load test as a measure of gastric interoception: Development of a two‐stage protocol and application to a healthy female population. PLoS ONE, 11(9), e0163574 10.1371/journal.pone.0163574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oudenhove, L. , Vandenberghe, J. , Dupont, P. , Geeraerts, B. , Vos, R. , Bormans, G. , … Tack, J. (2009). Cortical deactivations during gastric fundus distension in health: Visceral pain‐specific response or attenuation of «default mode» brain function? A H2 15O‐PET study. Neurogastroenterology and Motility, 21(3), 259–271. 10.1111/j.1365-2982.2008.01196.x [DOI] [PubMed] [Google Scholar]
- Vandenbergh, J. , Dupont, P. , Fischler, B. , Bormans, G. , Persoons, P. , Janssens, J. , & Tack, J. (2005). Regional brain activation during proximal stomach distention in humans: A positron emission tomography study. Gastroenterology, 128(3), 564–573. 10.1053/j.gastro.2004.11.054 [DOI] [PubMed] [Google Scholar]
- Varayil, J. E. , Ali, S. M. , Tachecí, I. , Květina, J. , Kopáčová, M. , Kuneš, M. , … Bureš, J. (2009). Electrogastrography in experimental pigs. Folia Gastroenterologica Et Hepatologica, 7(3–4), 98–104. [Google Scholar]
- Vasavid, P. , Chaiwatanarat, T. , Pusuwan, P. , Sritara, C. , Roysri, K. , Namwongprom, S. , … Gonlachanvit, S. (2014). Normal solid gastric emptying values measured by scintigraphy using asian‐style meal: A multicenter study in healthy volunteers. Journal of Neurogastroenterology and Motility, 20(3), 371–378. 10.5056/jnm13114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen, M. A. M. T. , Van Schelven, L. J. , Samsom, M. , & Smout, A. J. P. M. (1999). Pitfalls in the analysis of electrogastrographic recordings. Gastroenterology, 117(2), 453–460. 10.1053/gast.1999.0029900453 [DOI] [PubMed] [Google Scholar]
- Vianna, E. P. M. , Weinstock, J. , Elliott, D. , Summers, R. , & Tranel, D. (2006). Increased feelings with increased body signals. Social Cognitive and Affective Neuroscience, 1(1), 37–48. 10.1093/scan/nsl005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, B. A. , & Derbyshire, S. W. (2009). Visceral circuits and cingulate‐mediated autonomic functions In Vogt B. A. (Ed.), Cingulate neurobiology and disease (pp. 219–236). Oxford, UK: Oxford University Press. [Google Scholar]
- Waldhausen, J. H. T. , Shaffrey, M. E. , Skenderis, B. S. , Jones, R. S. , & Schirmer, B. D. (1990). Gastrointestinal myoelectric and clinical patterns of recovery after laparotomy. Annals of Surgery, 211(6), 777–785. 10.1097/00000658-199006000-00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, B. B. , & Sandman, C. A. (1977). Physiological response patterns in ulcer patients: Phasic and tonic components of the electrogastrogram. Psychophysiology, 14(4), 393–400. 10.1111/j.1469-8986.1977.tb02971.x [DOI] [PubMed] [Google Scholar]
- Walldén, J. , Lindberg, G. , Sandin, M. , Thörn, S.‐E. , & Wattwil, M. (2008). Effects of fentanyl on gastric myoelectrical activity: A possible association with polymorphisms of the mu‐opioid receptor gene? Acta Anaesthesiologica Scandinavica, 52(5), 708–715. 10.1111/j.1399-6576.2008.01624.x [DOI] [PubMed] [Google Scholar]
- Wang, G.‐J. , Tomasi, D. , Backus, W. , Wang, R. , Telang, F. , Geliebter, A. , … Volkow, N. D. (2008). Gastric distention activates satiety circuitry in the human brain. NeuroImage, 39(4), 1824–1831. 10.1016/j.neuroimage.2007.11.008 [DOI] [PubMed] [Google Scholar]
- White, E. H. (1964). Additional notes on gastrointestinal activity during avoidance behavior. Psychological Reports, 14(2), 343–347. 10.2466/pr0.1964.14.2.343 [DOI] [Google Scholar]
- Xing, J. , Qian, L. , & Chen, J. (2006). Experimental gastric dysrhythmias and its correlation with in vivo gastric muscle contractions. World Journal of Gastroenterology, 12(25), 3994–3998. 10.3748/wjg.v12.i25.3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, J. , & Chen, J. D. Z. (2013). Electrogastrography: Methodology, validation and applications. Journal of Neurogastroenterology and Motility, 19(1), 5–17. 10.5056/jnm.2013.19.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]


