The β‐arrestin proteins are key regulators of G protein‐coupled receptors, serving at least three distinct functions: inhibiting receptor signaling through G proteins, directing receptor trafficking from the cell surface after activation, and transmitting receptor‐initiated signals directly. How the two β‐arrestin proteins perform these many functions for hundreds of receptor types throughout the body, and specifically how β‐arrestin‐mediated signaling can be tuned to cellular conditions, remains an open question. Function‐based evidence and recent structure‐based evidence have suggested that patterns of receptor phosphorylation (“barcodes”) may be a critical determinant of β‐arrestin action. In this issue of EMBO Reports, Baidya and colleagues (Baidya et al, 2020a) report that specific receptor phosphorylation site clusters (“codes”) determine whether β‐arrestin 1 acts to promote or inhibit receptor activation of Erk mitogen‐activated protein kinases. They provide direct evidence for a functional barcode system by transferring inhibitory and stimulatory codes between receptors, suggesting future work to understand just how code site location in a receptor and its phosphorylation status can lead to very different functions of bound β‐arrestin proteins.
Subject Categories: Post-translational Modifications, Proteolysis & Proteomics; Signal Transduction
The β‐arrestin proteins are key regulators of G protein‐coupled receptors. A study in this issue reports that specific receptor phosphorylation codes determine whether β‐arrestin 1 acts to promote or inhibit receptor activation of Erk protein kinases.

G protein‐coupled receptors (GPCRs) utilize heterotrimeric G proteins to activate intracellular signaling pathways, but GPCRs also signaling via mechanisms that do not depend (or only indirectly depend) on G proteins (Pierce et al, 2002). When a GPCR is activated, it adopts a conformation that is recognized by G proteins, but also two other classes of proteins, the G protein‐coupled receptor kinases (GRKs) and arrestins. Upon binding to an activated GPCR, a GRK phosphorylates Ser/Thr in the receptor carboxyl tail and/or third intracellular loop. There are seven GRKs (GRKs 1‐7), and four of these regulate GPCRs in most of the body (beyond the visual system), namely GRKs 2, 3, 5, and 6 (Pierce et al, 2002). Once phosphorylated, activated receptors are bound by β‐arrestin proteins that prevent further G protein activation, link receptors to endocytotic pathways, and promote β‐arrestin‐dependent signaling via multiple partners. The two β‐arrestins (β‐Arr1 and β‐Arr2) regulate all GPCRs outside the visual system (Pierce et al, 2002).
The classic observation that receptor binding to β‐arrestins can be either transient or prolonged led to a receptor classification scheme where activated “class A” receptors with sparse or widely separated potential phospho‐sites bound β‐arrestin at the plasma membrane but released it as the receptor trafficked deeper into the cell, versus “class B” receptors with clustered potential phospho‐sites where receptor/β‐arrestin co‐trafficked deep into the cell (Oakley et al, 2000). Studies of many receptors using siRNA‐knockdown of GRK and β‐arrestin isoforms noted a general pattern that GRK2/3‐phosphorylation led to β‐Arr1‐mediated desensitization and trafficking, while GRK5/6‐phosphorylation most often led to β‐Arr2‐mediated signaling events; yet many exceptions to this rule were also noted. Nevertheless, a multitude of studies led to the overall hypothesis that β‐arrestin recruitment to distinct patterns of phosphorylated residues on receptors (“barcodes”) directed β‐arrestin function (Kim et al, 2005; Yang et al, 2017).
Structural studies of β‐arrestins bound to phosphorylated peptides and to phosphorylated receptors have illuminated how β‐arrestins bind to receptors and adopt an activated state (Lee et al, 2020). Phospho‐receptor/peptide binds the β‐arrestin N‐terminal domain and induces an active conformation that both binds tightly to the receptor core, but also presumably enables binding/activation of specific β‐arrestin partners. A recent structural description of how visual arrestin binds to phosphorylated rhodopsin (the visual GPCR) led to a model for specific receptor sequences that functioned as the actual phosphorylation‐dependent barcode sites (“phosphocodes”; Zhou et al, 2017).
It is in this context that the report by Baidya et al (2020a) illuminates how receptors engage β‐Arr1, based on the observation that many receptors (e.g., vasopressin V2 receptor and angiotensin II AT1a receptor) utilize β‐Arr1 to activate Erk (β‐Arr1 knockdown increases Erk activity), whereas for others (e.g., bradykinin B2 receptor), β‐Arr1 is inhibitory. Using this model, the authors use mutagenesis to probe the spatial requirements of putative phosphocode sites in the B2, V2, and AT1a receptors that enable receptor coupling through β‐Arr1 to Erk activation versus Erk inhibition. Remarkably, they were able to convert B2 receptor to β‐Arr1‐dependent Erk activation and AT1a receptor to β‐Arr1‐dependent Erk inhibition, by shifting the locations of phosphorylation sites within phosphocode regions. While it is routine to swap the entire carboxyl‐terminal tail of receptors in β‐arrestin‐binding studies (e.g., the class B V2 receptor tail onto the class A β2‐adrenergic receptor; Oakley et al, 2000), these new results refine our understanding of how flexible and responsive β‐arrestin proteins are to seemingly minor changes in the receptors to which they bind (Fig 1).
Figure 1. Receptor phosphorylation barcodes control β‐arrestin 1 signaling.
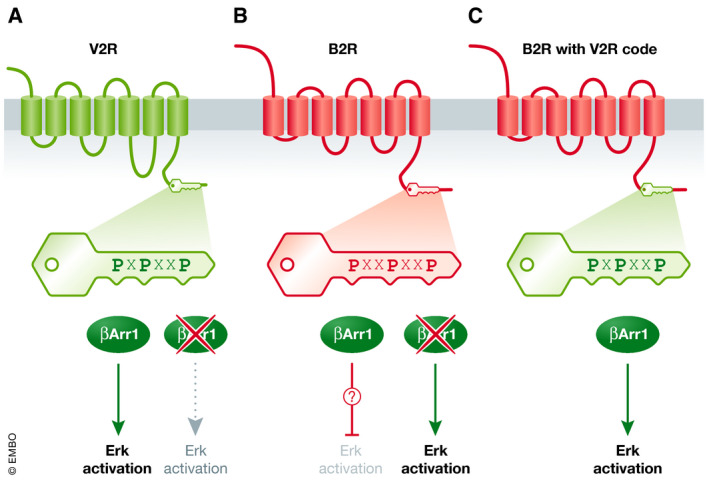
(A) The V2 vasopressin receptor (V2R) contains in its carboxyl‐terminal tail region clusters of Ser/Thr potential GRK phosphorylation sites (phosphocodes, green key). V2 activation stimulates Erk activity, and this activation is reduced when β‐Arr1 is knocked down (red “X”). (B) The B2 bradykinin receptor (B2R) has carboxyl‐terminal tail clusters of Ser/Thr potential GRK phosphorylation sites (phosphocodes, red key). B2 activation of Erk activity is enhanced when β‐Arr1 is knocked down (red “X”), indicating that B2R–β‐Arr1 inhibits Erk activation. (C) Mutagenesis of phosphocode residues in the B2 receptor to more closely match the spacing pattern in V2 receptor (green key) results in B2 receptor‐driven, β‐Arr1‐dependent activation of Erk activity. Thus, the phosphocode is a key that controls the signaling output of β‐Arr1 recruited to an activated receptor.
The authors also make use of an intrabody as a sensor to label a β‐Arr1 active conformation after activation of their mutant receptors, which demonstrates a shift in β‐Arr1 active conformation upon switching phosphocodes (Baidya et al, 2020a). A recent study by the same authors highlights the need for additional such sensors that detect further distinct active conformations (Baidya et al, 2020b).
Since β‐arrestins are general phospho‐GPCR binding proteins, the rules governing their recognition of specific phosphocodes and their translation of that into activity of bound signaling partners may be quite complex. Indeed, putative code sites very often are multiplexed/interleaved due to clustering of Ser/Thr residues in GPCRs; for example, a PxxPxxP motif in the V2 receptor is also a PxPxxP motif, due to additional phosphorylation sites within this short stretch of amino acids, and two PxxPxxP motifs in the B2 receptor overlap (i.e., PxxPxxPxxP) (Baidya et al, 2020a). Another complication is that due to ease of purification, β‐Arr1 has been used in most structural studies to date, while functional studies often focus on β‐Arr2, so how applicable these models are to GPCR recognition by β‐Arr2 remains to be determined.
Knowing that phosphocode clusters are important for directing β‐arrestin functions begs the question of which GRKs actually phosphorylate‐specific receptor sites, and in what precise pattern. Due to technical difficulty, few receptors have been examined for phosphorylation site preferences by specific GRKs, using mass spec or phosphosite antibodies, and only under limited conditions, e.g., (Mann et al, 2020). And like β‐arrestins, GRKs are capable of regulating hundreds of GPCRs, implying that this association is not driven by specificity of receptor–GRK interactions but instead by conformational recognition of the receptor active state. One major limitation of the current study is that phosphorylation of specific residues within the code sites was not assessed, and there is an implicit assumption that mutating or deleting residues to “transfer” a code pattern from one receptor to another preserves the ability to be phosphorylated in the same way as in the original receptor (by the same GRK, or to the same extent, etc.).
Nevertheless, the results by Baidya et al (2020a) provide evidence that “phosphocodes” exist functionally and provide a guide for future studies on these and other receptors, toward the goal of deriving robust general principles for how phosphocodes direct β‐arrestin functions. This is important conceptually and clinically, as much basic and translational effort is focused on understanding and utilizing the ability to alter the balance between receptor signaling via G proteins versus β‐arrestins (biased signaling; Kenakin, 2019). Looking further ahead, it seems likely that these general phosphocode functions will also be modulated by cellular state, through regulation of the availability of GRK activity or the pre‐assembly of β‐arrestin–partner complexes under specific conditions.
Acknowledgements
Work in the Premont laboratory is supported by the NIH grants R01 DK113159 and P01 HL075443.
EMBO Reports (2020) 21: e51249
See also: M Baidya et al (September 2020)
References
- Baidya M, Kumari P, Dwivedi‐Agnihotri H, Pandey S, Chaturvedi M, Stepniewski TM, Kawakami K, Cao Y, Laporte SA, Selent J et al (2020a) Key phosphorylation sites in GPCRs orchestrate the contribution of β‐Arrestin 1 in ERK1/2 activation EMBO Rep 21: e49886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidya M, Kumari P, Dwivedi‐Agnihotri H, Pandey S, Sokrat B, Sposini S, Chaturvedi M, Srivastava A, Roy D, Hanyaloglu AC et al (2020b) Genetically encoded intrabody sensors report the interaction and trafficking of β‐arrestin 1 upon activation of G‐protein‐coupled receptors J Biol Chem 295: 10153–10167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T (2019) Biased receptor signaling in drug discovery Pharmacol Rev 71: 267–315 [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ (2005) Functional antagonism of different G protein‐coupled receptor kinases for beta‐arrestin‐mediated angiotensin II receptor signaling Proc Natl Acad Sci U S A 102: 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Warne T, Nehme R, Pandey S, Dwivedi‐Agnihotri H, Chaturvedi M, Edwards PC, García‐Nafría J, Leslie AGW, Shukla AK et al (2020) Molecular basis of β‐arrestin coupling to formoterol‐bound β1‐adrenoceptor Nature 583: 862–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann A, Liebetrau S, Klima M, Dasgupta P, Massotte D, Schulz S (2020) Agonist‐induced phosphorylation bar code and differential post‐activation signaling of the delta opioid receptor revealed by phosphosite‐specific antibodies Sci Rep 10: 8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS (2000) Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein‐coupled receptors delineate two major classes of receptors J Biol Chem 275: 17201–17210 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven‐transmembrane receptors Nat Rev Mol Cell Biol 3: 639–650 [DOI] [PubMed] [Google Scholar]
- Yang Z, Yang F, Zhang D, Liu Z, Lin A, Liu C, Xiao P, Yu X, Sun JP (2017) Phosphorylation of G protein‐coupled receptors: from the barcode hypothesis to the flute model Mol Pharmacol 92: 201–210 [DOI] [PubMed] [Google Scholar]
- Zhou XE, He Y, De Waal PW, Gao X, Kang Y, Van Eps N, Yin Y, Pal K, Goswami D, White TA et al (2017) Identification of phosphorylation codes for arrestin recruitment by G protein‐coupled receptors Cell 170: 457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]


