Abstract
Gonadal hormones contribute to the sexual differentiation of brain and behavior throughout the lifespan, from initial neural patterning to “activation” of adult circuits. Sexual behavior is an ideal system in which to investigate the mechanisms underlying hormonal activation of neural circuits. Sexual behavior is a hormonally regulated, innate social behavior found across species. Although both sexes seek out and engage in sexual behavior, the specific actions involved in mating are sexually dimorphic. Thus, the neural circuits mediating sexual motivation and behavior in males and females are overlapping yet distinct. Furthermore, sexual behavior is strongly dependent on circulating gonadal hormones in both sexes. There has been significant recent progress on elucidating how gonadal hormones modulate physiological properties within sexual behavior circuits with consequences for behavior. Therefore, in this mini-review we review the neural circuits of male and female sexual motivation and behavior, from initial sensory detection of pheromones to the extended amygdala and on to medial hypothalamic nuclei and reward systems. We also discuss how gonadal hormones impact the physiology and functioning of each node within these circuits. By better understanding the myriad of ways in which gonadal hormones impact sexual behavior circuits, we can gain a richer and more complete appreciation for the neural substrates of complex behavior.
Keywords: reproductive behavior, gonadal hormones, sex hormones, activation, sex differences
Gonadal hormones play an essential role in the sexual differentiation of brain and behavior. Perinatal exposure to gonadal hormones guides neuronal growth, death, synaptogenesis, cytoarchitecture, chemoarchitecture, epigenetic modification, and many other brain characteristics to shape or “organize” sexually dimorphic neural circuits (1-6). Later exposure to gonadal hormones “activates” these circuits to promote expression of the relevant sex-typical behavior (5-7), and it is this deceptively simple concept we seek to spotlight in this mini-review. What does hormonal activation of a circuit mean at a mechanistic level? How is this implemented differently across circuit nodes and what is the consequence for behavior? We focus on sexual behavior as an ideal system in which to ask these questions: the behavior is ethologically relevant across species, easily studied in the laboratory, intensely dictated by hormonal status, and importantly—robustly expressed by both sexes. Therefore, herein we review key components of the neural circuitry underlying male and female sexual behaviors and highlight, whenever possible, recent advances in our understanding of the hormonal regulation of such circuits. We focus on literature from rodent models, due to their notable reliance on hormonal activation for sexual behavior and for the vast wealth of knowledge available from nearly a century of careful experimentation on these genetically tractable models.
The Hormonal Regulation of Sexual Behavior
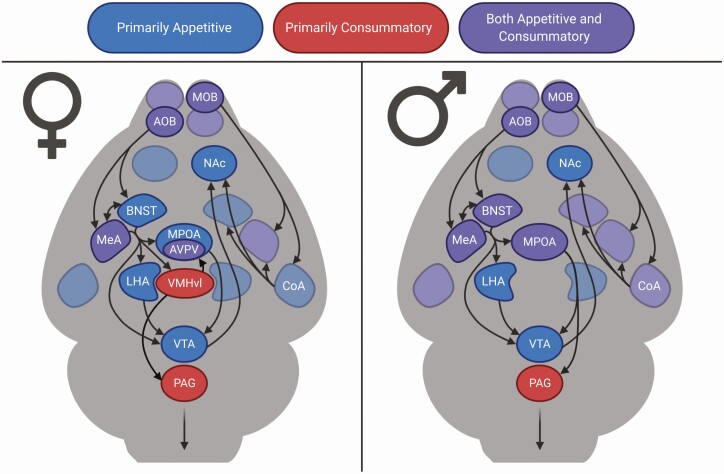
Sexual behaviors are often conceptualized into 2 categories: appetitive and consummatory (8). Appetitive sexual behavior entails actions that increase the likelihood of mating to occur and are thought to reflect sexual motivation. This includes approach, solicitation, or investigation of a potential mate and the exhibition of mate preference, or preference for an intact opposite-sex conspecific over a same-sex or gonadectomized conspecific. These behaviors can be displayed by both sexes with some species-specific differences (ie, specific solicitation behaviors may differ between sexes). On the other hand, consummatory sexual behavior entails the act of mating itself and is highly sexually dimorphic. In male rodents, this includes mounting and intromission, whereas in female rodents it is primarily adoption of the lordosis posture (stationary flexion of the spine and deflection of the tail permitting male intromission). Each of these aspects of sexual behavior is mediated by distinct but frequently overlapping neural substrates, which will be reviewed in the following sections (Fig. 1).
Figure 1.
Neural circuits of male and female sexual behavior. Regions are color-coded based on major contributions to either appetitive or consummatory aspects of sexual behavior.
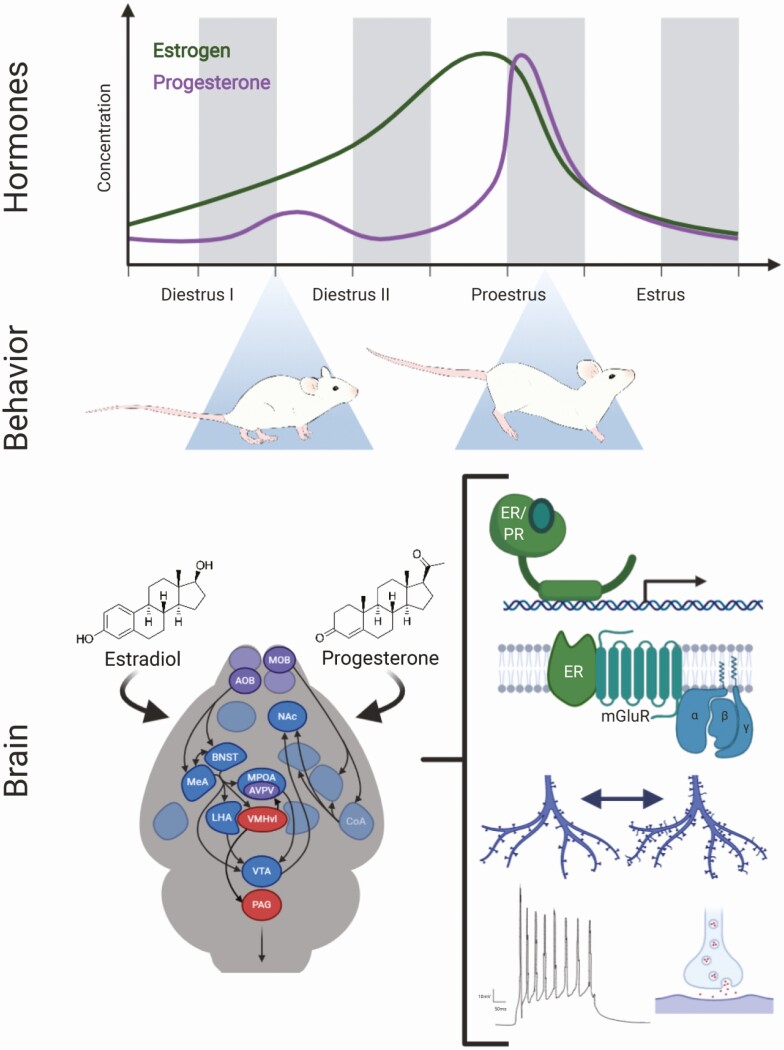
Sexual behavior in both sexes is strongly regulated by circulating concentrations of gonadal steroid hormones, including androgens (testosterone), estrogens (estradiol), and progesterone. In rodents, this hormonal regulation is perhaps most obvious in females across the 4- to 5-day estrous cycle (Fig. 2). Estradiol concentrations are low during diestrus but build to a peak by the afternoon of proestrus, which, in conjunction with a daily circadian signal, triggers ovulation (9, 10). This is quickly followed by a sharp peak in progesterone produced by the corpus luteum, which declines by the following morning (11-13). The sequential rise in estrogen followed by progesterone primes the female brain and physiology for sexual motivation and behavior (“in heat” or in estrus) (14). Outside of this window, female mice, rats, and hamsters will not be receptive toward a male and will actively reject mating attempts. Consequently, by co-opting the neuroendocrine signals of ovulation to regulate sexual behavior, the female conserves energetic resources by only mating when maximally fertile. Importantly, this is distinct from old-world primates. For females of these species, including women, sexual behavior is expressed across the ovulatory cycle and the influence of gonadal hormones on sexual behavior is comparatively subtle (15-18).
Figure 2.
Hormonal control of female sexual behavior. The sequential rise in estrogen followed by progesterone across the estrous cycle (top) causes female rodents to be sexually receptive near ovulation (middle). This is mediated by an array of neurophysiological changes to the brain induced by hormonal signaling (bottom). Gonadal steroid hormones signal both through nuclear receptors and membrane-bound receptors (bottom right). This signaling regulates gene expression, structural remodeling, neuronal activity, and changes in synaptic properties in a region-specific manner.
Male sexual behavior is also dependent on sufficient basal circulating gonadal hormones, primarily testosterone and its metabolites. Many of the activational effects of testosterone on male sexual behavior in rodents can be attributed to its conversion into estradiol by the enzyme aromatase (19-22), which is highly expressed along sexual behavior circuits (23, 24). However, for the full and complete expression of male sexual behavior in laboratory models, both androgen and estrogen receptor signaling is required (25-28) (but see (26) for discussion of species differences). Typical laboratory models do not exhibit hormonal cycles that greatly impact male sexual behavior, but many other species do display seasonal cycles of reproductive activity, with commensurate changes to neuroendocrine, behavioral, and sensory systems (29-31). Furthermore, social experience (eg, social dominance, stress) can modify the hormonal milieu, even within laboratory models (32-34). Exposure to a potential mate or to a social challenge elicits an acute and transient increase in testosterone above basal levels in males across species (35-38). Such socially induced testosterone pulses have been hypothesized to modify future behavior in several ways, including by promoting territory formation, promoting future winning (ie, the winner effect), modifying social vigilance, reducing anxiety, and potentially facilitating responses to the social situation through rapid, nongenomic actions (38-41). Thus, adults of both sexes can experience fluctuations in gonadal hormones that may impact brain and behavior.
The Main and Accessory Olfactory Systems
Animals rely on pheromone signaling to communicate social information essential for reproductive behavior. These chemosignals are detected by the complementary but distinct main and accessory olfactory systems (MOS, AOS) (42, 43). Within the MOS, sensory neurons in the main olfactory epithelium (MOE) detect volatile odorants and relay this information to the main olfactory bulb. Accordingly, the MOS is thought to be particularly important for initial approach behavior and inherent social attraction based on volatile cues (44, 45). On the other hand, within the AOS, sensory neurons of the vomeronasal organ (VNO) detect pheromones transmitted through close contact with a conspecific. This information is then conveyed to the accessory olfactory bulb, which sends projections to the extended amygdala that are considered particularly important for pheromonal elicitation of reproductive behavior and neuroendocrine responses (42, 46). Although these 2 systems are anatomically distinct and respond to different classes of pheromones, information from the MOS also reaches the extended amygdala through the cortical amygdala and a minor but direct projection from the main olfactory bulb (44, 47).
Both the MOS and AOS are essential for the complete and appropriate display of sexual behavior. Male and female pheromones elicit distinct sex-specific patterns of activation within both systems (47-50). Lesions or genetic disruption of either the MOE or VNO disrupt sociosexual behavior in both sexes (51-56). For example, genetic mutation of the ion channel TrpC2 abolishes pheromone signal transduction in the VNO. TrpC2-/- mice inappropriately mount same- and opposite-sex conspecifics at high levels (57-59), despite TrpC2-/- mice, or even animals with complete VNO lesions, retaining the ability to discriminate male versus female odorants through the MOS (54, 60-62). Thus, the AOS is considered particularly important for regulating the expression of specific social behaviors toward the appropriate target (eg, to mate or to attack) (46).
Recent work has shed light on how fluctuations in sex hormones across the estrous cycle shape sensory processing in the AOS. Estradiol regulates expression of ion channels within the VNO and rapidly modifies vomeronasal sensory neuron (VSN) responses to pheromones (63-65). Furthermore, Dey et al reported that moderate concentrations of progesterone (approximately that of diestrus, ~13 ng/mL) act to silence VSNs (66). Intriguingly, progesterone-mediated silencing was seen in VSNs sensitive to male pheromones but not VSNs that were sensitive to predator odor, revealing hormonal modulation specifically of socially relevant sensory input. However, this study did not test the effect of progesterone at high concentrations seen during late proestrus (~50 ng/mL (67-69)), so whether the peri-ovulatory progesterone surge might counterintuitively inhibit pheromone-sensing VSNs or whether this effect is dose-dependent remains to be tested. Regardless, changing concentrations of circulating estrogen and progesterone across the estrous cycle can clearly modulate the female’s earliest sensory detection of male cues. In males, testosterone has been shown to increase activation of both the AOS and MOS in response to female pheromones (70, 71), although there is little data available on the underlying molecular mechanisms mediating this effect (63).
The Extended Amygdala
The medial amygdala (MeA) is a major target of the AOS and minor target of the MOS that has been strongly implicated in mediating sexual behavior (47, 72). In particular, the posterodorsal subdivision of the MeA (MeApd) expresses a high density of sex hormone receptors and is well accepted to be activated during mating or by exposure to opposite-sex pheromones (51, 72-75). Indeed, recent work has demonstrated that neurons of the MeA differentially encode male versus female cues (76, 77), and that this separable encoding is shaped by experience (78). The MeApd appears to regulate aspects of both mate preference and consummatory sexual behavior. Lesions of the MeApd disrupt mate preference in both sexes (79-82). MeApd lesions also disrupt sexual behavior in males (83-86). In females, MeA lesion or MeA chemo-inhibition reduces but does not eliminate lordosis behavior (82, 87), and MeA lesions do not impact the amount of mounts or intromissions received in a mating assay (88). These data indicate that the MeA promotes lordosis responses but is not essential for its expression.
Recent studies targeting genetically identified MeApd subpopulations have highlighted the complex role of the MeApd in regulating multiple social behaviors. Disrupting oxytocin signaling in aromatase-expressing MeApd neurons eliminated mate preference in males (77). However, ablating these neurons did not impair sexual behavior in either sex, although it did impair aggressive behavior (89). GABAergic MeApd neurons can promote mounting or aggression depending on stimulation intensity (90). On the other hand, optogenetic inhibition of MeApd GABAergic neurons did not interrupt intromission, suggesting that while these neurons may facilitate mount initiation, they are not necessary for continued sexual behavior. Finally, chemogenetic stimulation of kisspeptin-expressing MeApd neurons in males promoted social investigation without impacting consummatory sexual behavior (91). Thus, it seems likely that there exists multiple parallel or combinatorial subcircuits involving the MeApd that guide expression of the appropriate social behavior to a given stimulus.
Another component of the “extended amygdala,” the bed nuclei of stria terminalis (BNST), is also implicated in the control of sexual behavior. The BNST receives direct and indirect input from the AOS, exhibits a high density of steroid hormone receptors, and contains numerous overlapping neuropeptide subpopulations (72, 92-95). The BNST contains spatially and genetically segregated neuronal subpopulations capable of driving either aversive or appetitive behavioral states. Of note, male cholecystokinin (CCK)-expressing medial BNST neurons are both preferentially activated by opposite-sex odorants and produce reinforcement when stimulated (93), which could conceivably contribute to the expression of mate preference. Lesions to the BNST largely delay or slow mating in males (96-102), with seemingly greater effects in naïve animals (101, 102). Indeed, aromatase-expressing neurons in the principal component of the BNST (BNSTprAro) were recently reported to exhibit distinct activity patterns in response to male versus female conspecifics in naïve males (103), distinguishing it from the MeA, which requires social experience to encode sex discrimination (78). Inhibiting or ablating these neurons eliminated mate preference and reduced consummatory sexual behavior and aggression, whereas stimulating these neurons (in line with the endogenous response to females) promoted mounting directed to male conspecifics (103). Thus, the authors propose that BNSTprAro neurons represent a neural substrate of sex recognition, vital information for the selection of appropriate social responses. Interestingly, this role seems unique to males, as BNSTprAro neurons in females do not show similar activity patterns or effects on behavior (103).
Gonadal hormones regulate several aspects of neuronal physiology within the extended amygdala. First, local replacement of testosterone or estradiol to the MeA in gonadectomized males facilitates expression of sexual behavior (104-108), indicating that hormonal signaling within this region promotes activation of sexual behavior circuits. Second, adult gonadal hormones support sexual dimorphism in regional volume and soma size within the MeA (109, 110). Third, fluctuations in estrogen and progesterone across the estrous cycle modulate synaptic and electrophysiological features of neurons within these regions (111-114). For example, estrogen has been reported to selectively regulate the excitability of afferents to the MeA in a source-specific manner (115). Similarly, the excitatory:inhibitory balance of inputs onto the MeApd varies across the estrous cycle (116). These changes could regulate the computational weight of various MeA inputs across the estrous cycle and bias sexual interest to the estrus period. Finally, gonadal hormones regulate expression of various neuropeptides within the MeA and BNST of both sexes (117-122), which likely further impacts neuromodulatory control of information processing. This includes expression of the neuropeptide CCK within the BNST (118), indicating that the reward-promoting BNSTcck population discussed above (93) is likely regulated to some degree by gonadal hormones. However, how these hormone-driven changes in neuronal physiology relate to observable changes in sociosexual behavior remain unclear.
Medial Hypothalamic Centers
Looking downstream of the extended amygdala, the medial preoptic area (MPOA) is essential for the display of male sexual behavior. This has been demonstrated by decades of lesion, stimulation, and pharmacological studies which have been excellently reviewed in detail elsewhere (28, 123). The MPOA receives a wide array of afferents and is thought to integrate information from both olfactory systems, hormonal state via rich expression of steroid receptors, and sensory input from the genitals (123). The MPOA comprises a heterogenous mix of cell types and exhibits distinct input/output patterns across different subregions (124-129), complicating functional dissection of this region. A recent study used fiber photometry to demonstrate esr1 (the gene for estrogen receptor α)-expressing MPOA neurons (MPOAesr1) are active during social investigation and that activity increased further during mounting. Optogenetic manipulation of MPOAesr1 neuronal activity bi-directionally regulated expression of mounting behavior (130). MPOAesr1 neurons also regulated expression of maternal behavior in both sexes, indicating that esr1 expression marks a broader MPOA population containing substrates of multiple hormonally regulated social behaviors. Interestingly, manipulating MPOAesr1 neuronal activity in males did not impact time spent investigating a female during mating assays, suggesting basic social interest is unaffected. Indeed, the MPOA’s role in sexual motivation was formerly controversial (131), as several studies reported that MPOA lesions did not impair male interest in female conspecifics (100, 132, 133). However, additional MPOA lesions studies across species have reported deficits in mate preference and pursuit behavior (134-139). Thus, it is now believed that the MPOA facilitates sexual motivation in addition to mediating consummatory sexual behavior in males.
On the other hand, the MPOA likely does not play a strong role in modulating lordosis behavior in females. Lesions of this region typically do not impair and may even promote expression of lordosis (81, 140-143). However, the MPOA does support sexual motivation in females, as MPOA lesions disrupt approach behavior and mate preference (81, 88, 143-147). Indeed, McHenry et al recently characterized a MPOA→ ventral tegmental area (VTA) circuit that promotes social interest in both sexes and is regulated by ovarian hormones across the estrous cycle (148). Female neurotensin-expressing MPOA neurons (MPOAnts) respond preferentially to male odors, and estradiol exposure enhanced both MPOAnts intrinsic excitability and MPOAnts responsiveness to male cues. Stimulation of either MPOAnts neurons or MPOAnts→VTA fibers was reinforcing in both sexes. Estradiol exposure (whether at proestrus or with exogenous treatment) enhanced this effect in females. Finally, bi-directionally manipulating MPOAnts activity regulated time spent investigating an opposite-sex conspecific and the expression of mate preference (148). These data support the role of the MPOA in appetitive sexual behavior in both sexes and provide a mechanism by which changes in ovarian hormones across the estrous cycle can gate female sexual motivation. Complementarily, estrogen can also act to inhibit female sexual behavior outside of the appropriate proestrus period. Briefly, estrogen acts in the arcuate nucleus via membrane-bound signaling to drive release of β-endorphin in the medial preoptic nucleus (MPN), which is embedded within the MPOA. This activation of µ opioid receptors in the MPN inhibits female sexual behavior. At proestrus, progesterone acts to de-activate MPN µ opioid receptors and thereby facilitate the transition to sexual receptivity (reviewed in (149, 150)). Thus, estrogen can both augment mate preference during proestrus and inhibit receptivity in the absence of the proestrus progesterone peak.
The ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) is essential for female lordosis behavior. Decades of work by Pfaff and colleagues characterized a lordosis reflex circuit with the VMH as a necessary and sufficient hormone-sensitive locus, which signals to midbrain premotor regions such as the periaqueductal gray (reviewed in (14, 149, 154, 155)). Local infusion of estradiol and progesterone into the VMHvl stimulates female sexual behavior (151-153). With advances in genetic access to specific cell types, progesterone receptor-expressing neurons in the VMHvl (VMHvlPR) have emerged as particularly essential for female sexual behavior (156). Recently, Inoue et al described a VMHvlPR→anteroventral periventricular nucleus (AVPV) circuit which is necessary for female receptivity and exhibits structural remodeling across the estrous cycle (157). Female VMHvlPR neurons are preferentially active during mating and when investigating males. Indeed, inhibiting VMHvlPR neurons reduced receptivity in hormonally primed females, but, surprisingly, stimulating VMHvlPR neurons failed to promote lordosis behavior in unprimed mice. Further investigation revealed that both exogenous estrogen exposure and the natural increase in estrogen during the estrous cycle increases the number of presynaptic terminals in the AVPV from VMHvlPR, with striking consequences for the functional connectivity between the VMHvl and AVPV. Inhibition of this pathway reduces lordosis behavior, indicating that the estrogenic gating of the VMHvlPR→AVPV circuit likely has behavioral consequences for limiting receptivity to behavioral estrus. Consistent with these data, the partially overlapping VMHvlesr1 neuronal population has been reported to contain mating- and fighting-activated subpopulations, with the mating-related but not fighting-related VMHvlesr1 subpopulation projecting strongly to the AVPV (158). Within the AVPV, there is further cell-type specificity: ablating AVPV tyrosine hydroxylase neurons did not impair female sexual behavior (159), whereas ablating AVPV kisspeptin neurons eliminated both mate preference and lordosis (160). AVPV kisspeptin neurons are themselves strongly regulated by ovarian hormones and play a prominent role in the neuroendocrine cascade regulating ovulation (161).
Reward Systems
It will surprise no one that sexual activity is rewarding and reinforcing. Activity of the mesolimbic dopamine system, namely dopamine release by the VTA into the nucleus accumbens (NAc), is thought to signal motivational salience of a stimulus, encode reward predictions, and facilitate reinforcement learning (162). The VTA receives significant input from the MPOA, MeA, and BNST in both sexes (163). Numerous groups have reported elevated dopamine release in the NAc upon presentation of a potential mate and during active mating in both males and females (164-172). With recent methodological advances enabling greater temporal resolution, we see escalating amount of dopamine release in a male as the mating behavioral suite progresses, with the highest release associated with ejaculation (173, 174). In female rats, dopamine release is dependent on the testing environment. If allowed space to retreat, female rats will pace the mating interaction such that she receives intromissions at an interval which will maximize her reproductive success (175-178). Accordingly, sexual reward and elevated dopamine release during mating is most robustly observed when mating proceeds at the female’s preferred pace, regardless of her display of lordosis behavior under nonpaced conditions (179-183). This is consistent with VTA dopamine release reflecting motivational state and sexual reward separate from motor actions.
VTA→NAc signaling likely supports expression of social interest and mate preference. Manipulating VTA→NAc neuronal activity bi-directionally modulates time spent investigating a conspecific (184). Furthermore, lesioning dopaminergic inputs to the NAc or blocking D1 receptor signaling in the NAc eliminated mate preference (185, 186). Conversely, stimulating dopamine release within the NAc rescued mate preference in TrpC2-/- mice, which have impaired pheromone detection (185). Another region of the ventral striatum, the medial olfactory tubercle, has been implicated in natural reinforcement and in encoding the innate hedonic valence of odorants (187, 188). Accordingly, lesions that include the medial olfactory tubercle or chemogenetic inhibition of the medial olfactory tubercle impair female mate preference for male odorants (189, 190).
Despite limited expression of steroid receptors in the VTA and NAc, estrogen is generally accepted to augment dopaminergic signaling in this pathway (191, 192). VTA dopaminergic neuron basal firing rate is enhanced and more dopamine released into the NAc during behavioral estrus (193-195). Several electrophysiological properties of NAc medium spiny neurons have been reported to either vary across the estrous cycle or to be rapidly modulated by estradiol exposure (192, 196, 197). Furthermore, like in many of the regions discussed above, estrogen exposure also regulates spine density in the NAc (198, 199). These effects seem to be region-specific within the striatum, as estrogenic effects on spine density and excitatory synaptic properties observed in the NAc were not seen in the caudate-putamen (197, 198). The effects of gonadal hormones on NAc physiology is not limited to females, as long-term treatment with androgens in males also modulates NAc dendritic spine density (200).
Another intriguing region that projects heavily to the VTA is the lateral hypothalamic area (LHA). This highly heterogeneous region modulates motivational drives relevant to many behaviors (201-203). The LHA has been implicated in both promoting and inhibiting sexual behavior in males. Serotonin is released into the LHA after ejaculation in males, and pharmacological elevation of serotonin within the LHA both attenuates dopamine release in the NAc in response to a female and inhibits sexual behavior (204, 205). Thus, serotonin signaling in the LHA may contribute to the postejaculatory refractory period, during which male sexual motivation is tightly suppressed. On the other hand, LHA neurons that express the neuropeptide hypocretin (hcrt, also known as orexin) promote goal-directed action in response to a wide array of stimuli (206-208). LHAhcrt neurons have been suggested to promote male sexual behavior based on increased activity during mating and pharmacological manipulation of hcrt signaling (209, 210). Interestingly, hcrt expression is also hormonally regulated in both sexes and varies across the estrous cycle (210-212), suggesting another avenue by which gonadal steroids can orchestrate sexual motivation.
Conclusion
Although for ease of explanation we have presented the above discussion as a forward flow of information from olfactory systems to the extended amygdala to the medial hypothalamus and reward systems, reality is not so straightforward. All the regions discussed above, and several others, send projections to each other, allowing for the possibility of feedback and crosstalk amongst systems. Furthermore, most of these regions have been implicated in the control of multiple social behaviors beyond sexual behavior, including territorial aggression, parental behavior, or maternal aggression. Indeed, based on neuroanatomical interconnections, strong steroid hormone receptor expression, and overlapping patterns of activation across social behaviors, the existence of a “social behavior network” was proposed (72). This network is highly conserved across taxa, providing a useful framework for comparative analysis (213, 214). This perspective has also proven useful for conceptualizing hormonal regulation of social behavior circuits. Through this lens, gonadal hormones act to tune connections and activity patterns across the social behavior network and thus shift the likelihood of a particular social response (72, 215). Indeed, as discussed above, gonadal hormones regulate a myriad of structural, electrophysiological, and genetic elements which converge to augment or attenuate circuit activity and behavioral output (Fig. 2). Recent and continued development of increasingly powerful tools is enabling unprecedented dissection of neuronal subcircuits with genetic precision. With this enhanced understanding of the neural circuits of behavior, we have a stronger foundation from which to probe the hormonal regulation of complex behavior.
Acknowledgments
We thank Sara Ponder for artistic assistance. Figures were created with BioRender.com.
Financial Support: This review was made possible by The Eunice Kennedy Shriver National Institute of Child Health and Human Development F32HD095597 (to K.J.).
Glossary
Abbreviations
- AOS
accessory olfactory system
- AVPV
anteroventral periventricular nucleus
- BNST
bed nuclei of stria terminalis
- CCK
cholecystokinin
- hcrt
hypocretin
- LHA
lateral hypothalamic area
- MeA
medial amygdala
- MeApd
posterodorsal subdivision of the medial amygdala
- MOE
main olfactory epithelium
- MOS
main olfactory system
- MPN
medial preoptic nucleus
- MPOA
medial preoptic area
- NAc
nucleus accumbens
- VMHvl
ventrolateral subdivision of the ventromedial hypothalamus
- VNO
vomeronasal organ
- VSN
vomeronasal sensory neuron
- VTA
ventral tegmental area
Additional Information
Disclosure Summary: The authors report no conflict of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Knoedler JR, Shah NM. Molecular mechanisms underlying sexual differentiation of the nervous system. Curr Opin Neurobiol. 2018;53:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forger NG, de Vries GJ, Breedlove SM. Sexual Differentiation of Brain and Behavior. In: Plant T, Zeleznik A, eds. Knobil and Neill's Physiology of Reproduction. 4th ed. Elsevier; 2015:2109-2155. [Google Scholar]
- 3. Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26(1):7-26. [DOI] [PubMed] [Google Scholar]
- 4. Wu MV, Shah NM. Control of masculinization of the brain and behavior. Curr Opin Neurobiol. 2011;21(1):116-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55(5):597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnold AP. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009;55(5):570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm Behav. 2008;53(2):307-11; author reply 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gotlieb N, Moeller J, Kriegsfeld LJ. Development and modulation of female reproductive function by Circadian signals. In: Wray S, Blackshaw S, eds. Developmental Neuroendocrinology. Cham: Springer; 2020:413-446. [Google Scholar]
- 10. Levine JE Neuroendocrine Control of the Ovarian Cycle of the Rat. 4th Ed. Elsevier; 2015:1199-1257. [Google Scholar]
- 11. Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96(1):219-226. [DOI] [PubMed] [Google Scholar]
- 12. Lukaszewska JH, Greenwald GS. Progesterone levels in the cyclic and pregnant hamster. Endocrinology. 1970;86(1):1-9. [DOI] [PubMed] [Google Scholar]
- 13. Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704-1708. [DOI] [PubMed] [Google Scholar]
- 14. Pfaus JG, Jones SL, Flanagan-Cato LM, Blaustein JD. Female sexual behavior. In: Plant T, Zeleznik A, eds. Knobil and Neill’s Physiology of Reproduction. 4th ed. Vol 2. Elsevier; 2015:2287-2370. [Google Scholar]
- 15. Baum MJ, Keverne EB, Everitt BJ, Herbert J, De Greef WJ. Effects of progesterone and estradiol on sexual attractivity of female rhesus monkeys. Physiol Behav. 1977;18(4):659-670. [DOI] [PubMed] [Google Scholar]
- 16. Wallen K. Sex and context: hormones and primate sexual motivation. Horm Behav. 2001;40(2):339-357. [DOI] [PubMed] [Google Scholar]
- 17. Roney JR, Simmons ZL. Hormonal predictors of sexual motivation in natural menstrual cycles. Horm Behav. 2013;63(4):636-645. [DOI] [PubMed] [Google Scholar]
- 18. Engelhardt A, Hodges JK, Niemitz C, Heistermann M. Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis). Horm Behav. 2005;47(2):195-204. [DOI] [PubMed] [Google Scholar]
- 19. Davidson JM. Effects of estrogen on the sexual behavior of male rats. Endocrinology. 1969;84(6):1365-1372. [DOI] [PubMed] [Google Scholar]
- 20. Södersten P. Estrogen-activated sexual behavior in male rats. Horm Behav. 1973;4(3):247-256. [DOI] [PubMed] [Google Scholar]
- 21. Ogawa S, Robbins A, Kumar N, Pfaff DW, Sundaram K, Bardin CW. Effects of testosterone and 7α-methyl-19-nortestosterone (MENT) on sexual and aggressive behaviors in two inbred strains of male mice. Horm. Behav. 1996;30(1):74-84. [DOI] [PubMed] [Google Scholar]
- 22. Beyer C, Moralí G, Naftolin F, Larsson K, Pérez-palacios. Effect of some antiestrogens and aromatase inhibitors on androgen induced sexual behavior in castrated male rats. Horm Behav. 1976;7(3):353-363. [DOI] [PubMed] [Google Scholar]
- 23. Balthazart J, Foidart A, Surlemont C, Harada N. Distribution of aromatase-immunoreactive cells in the mouse forebrain. Cell Tissue Res. 1991;263(1):71-79. [DOI] [PubMed] [Google Scholar]
- 24. Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370(1):71-84. [DOI] [PubMed] [Google Scholar]
- 25. Baum MJ, Vreeburg JT. Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science. 1973;182(4109):283-285. [DOI] [PubMed] [Google Scholar]
- 26. Hull EM, Wood RI, McKenna KE. Neurobiology of male sexual behavior. In: Neill JD, ed. Knobil and Neill’s Physiology of Reproduction. 3rd ed. Elsevier; 2006:1729-1824. [Google Scholar]
- 27. McGinnis MY, Mirth MC. Inhibition of cell nuclear androgen receptor binding and copulation in male rats by an antiandrogen, Sch 16423. Neuroendocrinology. 1986;43(1):63-68. [DOI] [PubMed] [Google Scholar]
- 28. Hull EM, Rodríguez-Manzo G. Male sexual behavior. In: Pfaff DW, Joëls M, eds. Hormones, Brain and Behavior. Vol 1. 3rd ed. Elsevier; 2017:1-57. [Google Scholar]
- 29. Walton JC, Weil ZM, Nelson RJ. Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol. 2011;32(3):303-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hazlerigg DG, Simonneaux V. Seasonal regulation of reproduction in mammals. In: Plant T, Zeleznik A, eds. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier; 2015:1575-1604. [Google Scholar]
- 31. Jennings KJ, Chasles M, Cho H, et al. The preoptic area and the RFamide-related peptide neuronal system gate seasonal changes in chemosensory processing. Integr Comp Biol. 2017;57(5):1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williamson CM, Lee W, Romeo RD, Curley JP. Social context-dependent relationships between mouse dominance rank and plasma hormone levels. Physiol Behav. 2017;171:110-119. [DOI] [PubMed] [Google Scholar]
- 33. Monder C, Sakai RR, Miroff Y, Blanchard DC, Blanchard RJ. Reciprocal changes in plasma corticosterone and testosterone in stressed male rats maintained in a visible burrow system: evidence for a mediating role of testicular 11 beta-hydroxysteroid dehydrogenase. Endocrinology. 1994;134(3):1193-1198. [DOI] [PubMed] [Google Scholar]
- 34. Machida T, Yonezawa Y, Noumura T. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm Behav. 1981;15(3):238-245. [DOI] [PubMed] [Google Scholar]
- 35. Gleason ED, Marler CA. Testosterone response to courtship predicts future paternal behavior in the California mouse, Peromyscus californicus. Horm Behav. 2010;57(2):147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wingfield JC, Hegner RE, Dufty AM, Ball GF. The “Challenge Hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 1990;136(6):829-846. [Google Scholar]
- 37. Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30(3):319-345. [DOI] [PubMed] [Google Scholar]
- 38. Nyby JG. Reflexive testosterone release: a model system for studying the nongenomic effects of testosterone upon male behavior. Front Neuroendocrinol. 2008;29(2):199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. James PJ, Nyby JG. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musculus). Physiol Behav. 2002;75(3):287-294. [DOI] [PubMed] [Google Scholar]
- 40. Marler CA, Trainor BC. The challenge hypothesis revisited: Focus on reproductive experience and neural mechanisms. Horm Behav. 2020;123:104645. [DOI] [PubMed] [Google Scholar]
- 41. Fuxjager MJ, Oyegbile TO, Marler CA. Independent and additive contributions of postvictory testosterone and social experience to the development of the winner effect. Endocrinology. 2011;152(9):3422-3429. [DOI] [PubMed] [Google Scholar]
- 42. Liberles SD. Mammalian pheromones. Annu Rev Physiol. 2014;76:151-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stowers L, Liberles SD. State-dependent responses to sex pheromones in mouse. Curr Opin Neurobiol. 2016;38:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hashikawa K, Hashikawa Y, Falkner A, Lin D. The neural circuits of mating and fighting in male mice. Curr Opin Neurobiol. 2016;38:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Root CM, Denny CA, Hen R, Axel R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 2014;515(7526):269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200(2):268-276. [DOI] [PubMed] [Google Scholar]
- 47. Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29(3):624-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434(7032):470-477. [DOI] [PubMed] [Google Scholar]
- 49. Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Bañón I, Crespo C, Insausti R, Martinez-Marcos A. Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25(7):2065-2080. [DOI] [PubMed] [Google Scholar]
- 50. Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci. 2007;26(2):463-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandez-Fewell GD, Meredith M. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J Neurosci. 1994;14(6):3643-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8(12):1660-1662. [DOI] [PubMed] [Google Scholar]
- 53. Fraser EJ, Shah NM. Complex chemosensory control of female reproductive behaviors. Plos One. 2014;9(2):e90368. doi:10.1371/journal.pone.0090368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23(2):521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006;31(4):315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123(4):669-682. [DOI] [PubMed] [Google Scholar]
- 57. Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295(5559):1493-1500. [DOI] [PubMed] [Google Scholar]
- 58. Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448(7157):1009-1014. [DOI] [PubMed] [Google Scholar]
- 59. Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99(9):6376-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Beny Y, Kimchi T. Conditioned odor aversion induces social anxiety towards females in wild-type and TrpC2 knockout male mice. Genes Brain Behav. 2016;15(8):722-732. [DOI] [PubMed] [Google Scholar]
- 61. Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24(42):9451-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex discrimination and odor preferences of female ferrets. Chem Senses. 2004;29(8):659-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cherian S, Wai Lam Y, McDaniels I, Struziak M, Delay RJ. Estradiol rapidly modulates odor responses in mouse vomeronasal sensory neurons. Neuroscience. 2014;269:43-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eckstein E, Pyrski M, Pinto S, Freichel M, Vennekens R, Zufall F. Cyclic regulation of Trpm4 expression in female vomeronasal neurons driven by ovarian sex hormones. Mol Cell Neurosci. 2020;105:103495. doi:10.1016/j.mcn.2020.103495 [DOI] [PubMed] [Google Scholar]
- 65. Kanageswaran N, Nagel M, Scholz P, Mohrhardt J, Gisselmann G, Hatt H. Modulatory effects of sex steroids progesterone and estradiol on odorant evoked responses in olfactory receptor neurons. Plos One. 2016;11(8):e0159640. doi:10.1371/journal.pone.0159640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dey S, Chamero P, Pru JK, et al. Cyclic regulation of sensory perception by a female hormone alters behavior. Cell. 2015;161(6):1334-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Michael SD. Plasma prolactin and progesterone during the estrous cycle in the mouse. Proc Soc Exp Biol Med. 1976;153(2):254-257. [DOI] [PubMed] [Google Scholar]
- 68. DeLeon DD, Zelinski-Wooten MB, Barkley MS. Hormonal basis of variation in oestrous cyclicity in selected strains of mice. J Reprod Fertil. 1990;89(1):117-126. [DOI] [PubMed] [Google Scholar]
- 69. Bailey KJ. Diurnal progesterone rhythms in the female mouse. J Endocrinol. 1987;112(1):15-21. [DOI] [PubMed] [Google Scholar]
- 70. Paredes RG, Lopez ME, Baum MJ. Testosterone augments neuronal Fos responses to estrous odors throughout the vomeronasal projection pathway of gonadectomized male and female rats. Horm Behav. 1998;33(1):48-57. [DOI] [PubMed] [Google Scholar]
- 71. Kelliher KR, Liu YC, Baum MJ, Sachs BD. Neuronal Fos activation in olfactory bulb and forebrain of male rats having erections in the presence of inaccessible estrous females. Neuroscience. 1999;92(3):1025-1033. [DOI] [PubMed] [Google Scholar]
- 72. Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242-257. [DOI] [PubMed] [Google Scholar]
- 73. Choi GB, Dong HW, Murphy AJ, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46(4):647-660. [DOI] [PubMed] [Google Scholar]
- 74. Minerbo G, Albeck D, Goldberg E, et al. Activity of peptidergic neurons in the amygdala during sexual behavior in the male rat. Exp Brain Res. 1994;97(3):444-450. [DOI] [PubMed] [Google Scholar]
- 75. Kollack SS, Newman SW. Mating behavior induces selective expression of Fos protein within the chemosensory pathways of the male Syrian hamster brain. Neurosci Lett. 1992;143(1-2):223-228. [DOI] [PubMed] [Google Scholar]
- 76. Bergan JF, Ben-Shaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. Elife. 2014;3:e02743. doi:10.7554/eLife.02743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yao S, Bergan JF, Lanjuin A, Dulac C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife 2017;6:1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Mathis A, Grewe BF, et al. Neuronal representation of social information in the medial Amygdala of awake behaving mice. Cell 2017;171(5):1176-1190.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24(12):3541-3552. [DOI] [PubMed] [Google Scholar]
- 80. Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;113(2):345-357. [DOI] [PubMed] [Google Scholar]
- 81. Xiao K, Kondo Y, Sakuma Y. Differential regulation of female rat olfactory preference and copulatory pacing by the lateral septum and medial preoptic area. Neuroendocrinology. 2005;81(1):56-62. [DOI] [PubMed] [Google Scholar]
- 82. DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav. 2012;105(2):554-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol Behav. 1992;51(5):939-943. [DOI] [PubMed] [Google Scholar]
- 84. Kondo Y, Arai Y. Functional association between the medial amygdala and the medial preoptic area in regulation of mating behavior in the male rat. Physiol Behav. 1995;57(1):69-73. [DOI] [PubMed] [Google Scholar]
- 85. Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210(4469):557-560. [DOI] [PubMed] [Google Scholar]
- 86. Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240(1):27-41. [DOI] [PubMed] [Google Scholar]
- 87. McCarthy EA, Maqsudlu A, Bass M, Georghiou S, Cherry JA, Baum MJ. DREADD-induced silencing of the medial amygdala reduces the preference for male pheromones and the expression of lordosis in estrous female mice. Eur J Neurosci. 2017;46(4):2035-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guarraci FA, Megroz AB, Clark AS. Paced mating behavior in the female rat following lesions of three regions responsive to vaginocervical stimulation. Brain Res. 2004;999(1):40-52. [DOI] [PubMed] [Google Scholar]
- 89. Unger EK, Burke KJ Jr, Yang CF, Bender KJ, Fuller PM, Shah NM. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 2015;10(4):453-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158(6):1348-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Adekunbi DA, Li XF, Lass G, et al. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J Neuroendocrinol. 2018;30(3):e12572. doi:10.1111/jne.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21(4):450-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li SB, Malenka RC, de Lecea L. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat Neurosci. 2018;21(8):1084-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ch’ng S, Fu J, Brown RM, McDougall SJ, Lawrence AJ. The intersection of stress and reward: BNST modulation of aversive and appetitive states. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2018;87(Pt A):108-125. [DOI] [PubMed] [Google Scholar]
- 95. Welch JD, Kozareva V, Ferreira A, Vanderburg C, Martin C, Macosko EZ. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell. 2019;177(7):1873-1887.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Emery DE, Sachs BD. Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol Behav. 1976;17(5):803-806. [DOI] [PubMed] [Google Scholar]
- 97. Valcourt RJ, Sachs BD. Penile reflexes and copulatory behavior in male rats following lesions in the bed nucleus of the stria terminalis. Brain Res Bull. 1979;4(1):131-133. [DOI] [PubMed] [Google Scholar]
- 98. Lehman MN, Powers JB, Winans SS. Stria terminalis lesions alter the temporal pattern of copulatory behavior in the male golden hamster. Behav Brain Res. 1983;8(1):109-128. [DOI] [PubMed] [Google Scholar]
- 99. Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23(3):181-195. [DOI] [PubMed] [Google Scholar]
- 100. Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17(13):5245-5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Claro F, Segovia S, Guilamón A, Del Abril A. Lesions in the medial posterior region of the BST impair sexual behavior in sexually experienced and inexperienced male rats. Brain Res Bull. 1995;36(1):1-10. [DOI] [PubMed] [Google Scholar]
- 102. Been LE, Petrulis A. Lesions of the posterior bed nucleus of the stria terminalis eliminate opposite-sex odor preference and delay copulation in male Syrian hamsters: role of odor volatility and sexual experience. Eur J Neurosci. 2010;32(3):483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bayless DW, Yang T, Mason MM, Susanto AAT, Lobdell A, Shah NM. Limbic neurons shape sex recognition and social behavior in sexually naive males. Cell 2019;0(0):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wood RI. Estradiol, but not dihydrotestosterone, in the medial amygdala facilitates male hamster sex behavior. Physiol Behav. 1996;59(4-5):833-841. [DOI] [PubMed] [Google Scholar]
- 105. Coolen LM, Wood RI. Testosterone stimulation of the medial preoptic area and medial amygdala in the control of male hamster sexual behavior: redundancy without amplification. Behav Brain Res. 1999;98(1):143-153. [DOI] [PubMed] [Google Scholar]
- 106. Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behaviour in the male Syrian hamster: role of the medial amygdaloid nucleus. Neuroscience. 1997;78(4):1027-1035. [DOI] [PubMed] [Google Scholar]
- 107. Huddleston GG, Michael RP, Zumpe D, Clancy AN. Estradiol in the male rat amygdala facilitates mounting but not ejaculation. Physiol Behav. 2003;79(2):239-246. [DOI] [PubMed] [Google Scholar]
- 108. Bialy M, Sachs BD. Androgen implants in medial amygdala briefly maintain noncontact erection in castrated male rats. Horm Behav. 2002;42(3):345-355. [DOI] [PubMed] [Google Scholar]
- 109. Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138(3):997-1005. [DOI] [PubMed] [Google Scholar]
- 110. Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43(2):336-346. [DOI] [PubMed] [Google Scholar]
- 111. Hansberg-Pastor V, González-Arenas A, Piña-Medina AG, Camacho-Arroyo I. Sex hormones regulate cytoskeletal proteins involved in brain plasticity. Front Psychiatry. 2015;6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gomez DM, Newman SW. Medial nucleus of the amygdala in the adult Syrian hamster: a quantitative Golgi analysis of gonadal hormonal regulation of neuronal morphology. Anat Rec. 1991;231(4):498-509. [DOI] [PubMed] [Google Scholar]
- 113. Frankiensztajn LM, Gur-Pollack R, Wagner S. A combinatorial modulation of synaptic plasticity in the rat medial amygdala by oxytocin, urocortin3 and estrogen. Psychoneuroendocrinology. 2018;92:95-102. [DOI] [PubMed] [Google Scholar]
- 114. Zancan M, da Cunha RSR, Schroeder F, Xavier LL, Rasia-Filho AA. Remodeling of the number and structure of dendritic spines in the medial amygdala: From prepubertal sexual dimorphism to puberty and effect of sexual experience in male rats. Eur J Neurosci. 2018;48(2):1851-1865. [DOI] [PubMed] [Google Scholar]
- 115. Yoshida M, Suga S, Sakuma Y. Estrogen reduces the excitability of the female rat medial amygdala afferents from the medial preoptic area but not those from the lateral septum. Exp Brain Res. 1994;101(1):1-7. [DOI] [PubMed] [Google Scholar]
- 116. Dalpian F, Rasia-Filho AA, Calcagnotto ME. Sexual dimorphism, estrous cycle and laterality determine the intrinsic and synaptic properties of medial amygdala neurons in rat. J. Cell Sci. 2019;132(9):jcs227793. 10.1242/jcs.227793 [DOI] [PubMed] [Google Scholar]
- 117. Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579(2):321-326. [DOI] [PubMed] [Google Scholar]
- 118. Simerly RB, Swanson LW. Castration reversibly alters levels of cholecystokinin immunoreactivity within cells of three interconnected sexually dimorphic forebrain nuclei in the rat. Proc Natl Acad Sci U S A. 1987;84(7):2087-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. De Vries GJ, al-Shamma HA. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol. 1990;21(5):686-693. [DOI] [PubMed] [Google Scholar]
- 120. Wang Z, De Vries GJ. Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster). Brain Res. 1993;631(1):156-160. [DOI] [PubMed] [Google Scholar]
- 121. Wang Z, De Vries GJ. Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. J Neuroendocrinol. 1995;7(11):827-831. [DOI] [PubMed] [Google Scholar]
- 122. Malsbury CW, McKay K. Neurotrophic effects of testosterone on the medial nucleus of the amygdala in adult male rats. J Neuroendocrinol. 1994;6(1):57-69. [DOI] [PubMed] [Google Scholar]
- 123. Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52(1):45-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Moffitt JR, Bambah-Mukku D, Eichhorn SW, et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science (80-.). 2018;362(6416):eaau5324. 10.1126/science.aau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Simerly RB, Gorski RA, Swanson LW. Neurotransmitter specificity of cells and fibers in the medial preoptic nucleus: an immunohistochemical study in the rat. J Comp Neurol. 1986;246(3):343-363. [DOI] [PubMed] [Google Scholar]
- 126. Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246(3):312-342. [DOI] [PubMed] [Google Scholar]
- 127. Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270(2):209-242. [DOI] [PubMed] [Google Scholar]
- 128. Coolen LM, Peters HJ, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J Comp Neurol. 1998;397(3):421-435. [DOI] [PubMed] [Google Scholar]
- 129. Tsuneoka Y, Yoshida S, Takase K, Oda S, Kuroda M, Funato H. Neurotransmitters and neuropeptides in gonadal steroid receptor-expressing cells in medial preoptic area subregions of the male mouse. Sci Rep. 2017;7(1):9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wei YC, Wang SR, Jiao ZL, et al. Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat Commun. 2018;9(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14(2):217-232. [DOI] [PubMed] [Google Scholar]
- 132. Hansen S, af Hagelsrum LJ. Emergence of displacement activities in the male rat following thwarting of sexual behavior. Behav Neurosci. 1984;98(5):868-883. [DOI] [PubMed] [Google Scholar]
- 133. Heimer L, Larsson K. Impairment of mating behavior in male rats following lesions in the preoptic-anterior hypothalamic continuum. Brain Res. 1967;3(3):248-263. [Google Scholar]
- 134. Edwards DA, Einhorn LC. Preoptic and midbrain control of sexual motivation. Physiol Behav. 1986;37(2):329-335. [DOI] [PubMed] [Google Scholar]
- 135. Edwards DA, Walter B, Liang P. Hypothalamic and olfactory control of sexual behavior and partner preference in male rats. Physiol Behav. 1996;60(5):1347-1354. [DOI] [PubMed] [Google Scholar]
- 136. Kindon HA, Baum MJ, Paredes RJ. Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm Behav. 1996;30(4):514-527. [DOI] [PubMed] [Google Scholar]
- 137. Paredes RG, Highland L, Karam P. Socio-sexual behavior in male rats after lesions of the medial preoptic area: evidence for reduced sexual motivation. Brain Res. 1993;618(2):271-276. [DOI] [PubMed] [Google Scholar]
- 138. Paredes RG, Tzschentke T, Nakach N. Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res. 1998;813(1):1-8. [DOI] [PubMed] [Google Scholar]
- 139. Paredes RG, Baum MJ. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. J Neurosci. 1995;15(10):6619-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Malsbury CW, Pfaff DW, Malsbury AM. Suppression of sexual receptivity in the female hamster: neuroanatomical projections from preoptic and anterior hypothalamic electrode sites. Brain Res. 1980;181(2):267-284. [DOI] [PubMed] [Google Scholar]
- 141. Powers B, Valenstein ES. Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science. 1972;175(4025):1003-1005. [DOI] [PubMed] [Google Scholar]
- 142. Hoshina Y, Takeo T, Nakano K, Sato T, Sakuma Y. Axon-sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behav Brain Res. 1994;61(2):197-204. [DOI] [PubMed] [Google Scholar]
- 143. Spiteri T, Ogawa S, Musatov S, Pfaff DW, Agmo A. The role of the estrogen receptor α in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav Brain Res. 2012;230(1):11-20. [DOI] [PubMed] [Google Scholar]
- 144. Whitney JF. Effect of medial preoptic lesions on sexual behavior of female rats is determined by test situation. Behav Neurosci. 1986;100(2):230-235. [DOI] [PubMed] [Google Scholar]
- 145. Clemens LG, Yang L-Y. MPOA lesions affect female pacing of copulation in rats. Behav. Neurosci. 2000;114(6):1191-1202. [PubMed] [Google Scholar]
- 146. Martinez LA, Petrulis A. The medial preoptic area is necessary for sexual odor preference, but not sexual solicitation, in female Syrian hamsters. Horm Behav. 2013;63(4):606-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Guarraci FA, Clark AS. Ibotenic acid lesions of the medial preoptic area disrupt the expression of partner preference in sexually receptive female rats. Brain Res. 2006;1076(1):163-170. [DOI] [PubMed] [Google Scholar]
- 148. McHenry JA, Otis JM, Rossi MA, et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci. 2017;20(3):449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Micevych PE, Meisel RL. Integrating neural circuits controlling female sexual behavior. Front Syst Neurosci. 2017;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Micevych PE, Sinchak K. Extranuclear signaling by ovarian steroids in the regulation of sexual receptivity. Horm Behav. 2018;104:4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogen-primed rats. Endocrinology. 1983;113(2):797-804. [DOI] [PubMed] [Google Scholar]
- 152. Rubin BS, Barfield RJ. Priming of estrous responsiveness by implants of 17 beta-estradiol in the ventromedial hypothalamic nucleus of female rats. Endocrinology. 1980;106(2):504-509. [DOI] [PubMed] [Google Scholar]
- 153. Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37(3):218-224. [DOI] [PubMed] [Google Scholar]
- 154. Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res. 1998;92(2):169-180. [DOI] [PubMed] [Google Scholar]
- 155. Pfaff DW, Kow LM, Loose MD, Flanagan-Cato LM. Reverse engineering the lordosis behavior circuit. Horm Behav. 2008;54(3):347-354. [DOI] [PubMed] [Google Scholar]
- 156. Yang CF, Chiang MC, Gray DC, et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153(4):896-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Inoue S, Yang R, Tantry A, et al. Periodic remodeling in a neural circuit governs timing of female sexual behavior. Cell. 2019;179(6):1393-1408.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hashikawa K, Hashikawa Y, Tremblay R, et al. Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat Neurosci. 2017;20(11):1580-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Scott N, Prigge M, Yizhar O, Kimchi T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature. 2015;525(7570):519-522. [DOI] [PubMed] [Google Scholar]
- 160. Hellier V, Brock O, Candlish M, et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat Commun. 2018;9(1):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Smith JT. Sex steroid regulation of kisspeptin circuits. In: Kauffman AS, Smith J, eds. Kisspeptin Signaling in Reproductive Biology. New York: Springer; 2013:275-295. [DOI] [PubMed] [Google Scholar]
- 162. Watabe-Uchida M, Eshel N, Uchida N. neural circuitry of reward prediction error. Annu Rev Neurosci. 2017;40:373-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chung AS, Miller SM, Sun Y, Xu X, Zweifel LS. Sexual congruency in the connectome and translatome of VTA dopamine neurons. Sci Rep. 2017;7(1):11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Pfaus JG, Damsma G, Nomikos GG, et al. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530(2):345-348. [DOI] [PubMed] [Google Scholar]
- 165. Mas M, Gonzalez-Mora JL, Louilot A, Solé C, Guadalupe T. Increased dopamine release in the nucleus accumbens of copulating male rats as evidenced by in vivo voltammetry. Neurosci Lett. 1990;110(3):303-308. [DOI] [PubMed] [Google Scholar]
- 166. Pleim ET, Matochik JA, Barfield RJ, Auerbach SB. Correlation of dopamine release in the nucleus accumbens with masculine sexual behavior in rats. Brain Res. 1990;524(1):160-163. [DOI] [PubMed] [Google Scholar]
- 167. Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106(1):181-191. [DOI] [PubMed] [Google Scholar]
- 168. Wenkstern D, Pfaus JG, Fibiger HC. Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Res. 1993;618(1):41-46. [DOI] [PubMed] [Google Scholar]
- 169. Robinson DL, Phillips PE, Budygin EA, Trafton BJ, Garris PA, Wightman RM. Sub-second changes in accumbal dopamine during sexual behavior in male rats. Neuroreport. 2001;12(11):2549-2552. [DOI] [PubMed] [Google Scholar]
- 170. Goto A, Nakahara I, Yamaguchi T, et al. Circuit-dependent striatal PKA and ERK signaling underlies rapid behavioral shift in mating reaction of male mice. Proc Natl Acad Sci U S A. 2015;112(21):6718-6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693(1-2):21-30. [DOI] [PubMed] [Google Scholar]
- 172. Meisel RL, Camp DM, Robinson TE. A microdialysis study of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behav Brain Res. 1993;55(2):151-157. [DOI] [PubMed] [Google Scholar]
- 173. Sun F, Zeng J, Jing M, et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell. 2018;174(2):481-496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Sun F, Zhou J, Dai B, et al. New and improved GRAB fluorescent sensors for monitoring dopaminergic activity in vivo. bioRxiv. Preprint posted online March 31, 2020. 10.1101/2020.03.28.013722. [DOI]
- 175. Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J Reprod Fertil. 1990;90(2):375-385. [DOI] [PubMed] [Google Scholar]
- 176. Coopersmith C, Erskine MS. Influence of paced mating and number of intromissions on fertility in the laboratory rat. J Reprod Fertil. 1994;102(2):451-458. [DOI] [PubMed] [Google Scholar]
- 177. McClintock MK, Adler NT. The role of the female during copulation in wild and domestic Norway rats (Rattus Norvegicus). Behaviour 1978;67(1-2):67-95. [Google Scholar]
- 178. Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav Neurosci. 1985;99(1):151-161. [DOI] [PubMed] [Google Scholar]
- 179. Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109(2):354-365. [DOI] [PubMed] [Google Scholar]
- 180. Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 2003;18(7):1997-2001. [DOI] [PubMed] [Google Scholar]
- 181. Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003;43(4):503-507. [DOI] [PubMed] [Google Scholar]
- 182. Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21(9):3236-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav Neurosci. 1997;111(1):123-128. [DOI] [PubMed] [Google Scholar]
- 184. Gunaydin LA, Grosenick L, Finkelstein JC, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Beny-Shefer Y, Zilkha N, Lavi-Avnon Y, et al. Nucleus accumbens dopamine signaling regulates sexual preference for females in male mice. Cell Rep. 2017;21(11):3079-3088. [DOI] [PubMed] [Google Scholar]
- 186. DiBenedictis BT, Olugbemi AO, Baum MJ, Cherry JA. 6-Hydroxydopamine lesions of the anteromedial ventral striatum impair opposite-sex urinary odor preference in female mice. Behav Brain Res. 2014;274:243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25(20):5061-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Fitzgerald BJ, Richardson K, Wesson DW. Olfactory tubercle stimulation alters odor preference behavior and recruits forebrain reward and motivational centers. Front Behav Neurosci. 2014;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Dibenedictis BT, Olugbemi AO, Baum MJ, Cherry JA. DREADD-induced silencing of the medial olfactory tubercle disrupts the preference of female mice for opposite-sex chemosignals. eNeuro 2015;2(5):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Agustín-Pavón C, Martínez-García F, Lanuza E. Focal lesions within the ventral striato-pallidum abolish attraction for male chemosignals in female mice. Behav Brain Res. 2014;259:292-296. [DOI] [PubMed] [Google Scholar]
- 191. Becker JB. Sex differences in addiction. Dialogues Clin Neurosci. 2016;18(4):395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Meitzen J, Meisel RL, Mermelstein PG. Sex differences and the effects of estradiol on striatal function. Curr Opin Behav Sci. 2018;23:42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Calipari ES, Juarez B, Morel C, et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8:13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014;135:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhang D, Yang S, Yang C, Jin G, Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl). 2008;199(4):625-635. [DOI] [PubMed] [Google Scholar]
- 196. Proaño SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J. Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol. 2018;120(3):1356-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Krentzel AA, Barrett LR, Meitzen J. Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen. J Neurophysiol. 2019;122(3):1213-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215(3-4):187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2015;220(4):2415-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Wallin-Miller K, Li G, Kelishani D, Wood RI. Anabolic-androgenic steroids decrease dendritic spine density in the nucleus accumbens of male rats. Neuroscience. 2016;330:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Bonnavion P, Mickelsen LE, Fujita A, de Lecea L, Jackson AC. Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J. Physiol. 2016;58(12):7250-7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Tyree SM, de Lecea L. Lateral hypothalamic control of the ventral tegmental area: reward evaluation and the driving of motivated behavior. Front Syst Neurosci. 2017;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19(2):198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lorrain DS, Matuszewich L, Friedman RD, Hull EM. Extracellular serotonin in the lateral hypothalamic area is increased during the postejaculatory interval and impairs copulation in male rats. J Neurosci. 1997;17(23):9361-9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lorrain DS, Riolo JV, Matuszewich L, Hull EM. Lateral hypothalamic serotonin inhibits nucleus accumbens dopamine: implications for sexual satiety. J Neurosci. 1999;19(17):7648-7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Graebner AK, Iyer M, Carter ME. Understanding how discrete populations of hypothalamic neurons orchestrate complicated behavioral states. Front Syst Neurosci. 2015;9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Nevárez N, de Lecea L. Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation. F1000Research 2018;7(0):1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Jennings KJ, de Lecea L. Hypocretins (Orexins): twenty years of dissecting arousal circuits. In: The Orexin/Hypocretin System. Elsevier; 2019:1-29. [Google Scholar]
- 209. Gulia KK, Mallick HN, Kumar VM. Orexin A (hypocretin-1) application at the medial preoptic area potentiates male sexual behavior in rats. Neuroscience. 2003;116(4):921-923. [DOI] [PubMed] [Google Scholar]
- 210. Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27(11):2837-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D. Orexin A and B levels in the hypothalamus of female rats: the effects of the estrous cycle and age. Eur J Endocrinol. 2004;150(5):737-742. [DOI] [PubMed] [Google Scholar]
- 212. Silveyra P, Catalano PN, Lux-Lantos V, Libertun C. Impact of proestrous milieu on expression of orexin receptors and prepro-orexin in rat hypothalamus and hypophysis: actions of Cetrorelix and Nembutal. Am J Physiol Endocrinol Metab. 2007;292(3):E820-E828. [DOI] [PubMed] [Google Scholar]
- 213. Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48(1):11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336(6085): 1154-1157. [DOI] [PubMed] [Google Scholar]
- 215. Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front Neuroendocrinol. 2009;30(4):429-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.