Abstract
Background
The Selvester QRS score can identify the presence and extent of myocardial scar in ischemic and nonischemic cardiomyopathy, but its performance in patients with hypertrophic cardiomyopathy (HCM) has not been assessed.
Methods
Consecutive patients with HCM referred to our hospital between January 2012 and July 2016 were prospectively enrolled. All patients underwent cardiac magnetic resonance (CMR) and 12‐lead electrocardiography. The Selvester QRS score was used to evaluate the presence and extent of myocardial scarring, and the results were compared with that obtained with the gold standard—late gadolinium enhancement (LGE) on CMR.
Results
A total of 135 HCM patients were enrolled. LGE was present in 93 of 135 (69%) patients. The median LGE mass was 5 (0–14) g, and the median proportion of total left ventricular mass showing LGE was 4% (0%–10%). A total of 92 patients had Selvester score ≥1. The highest score recorded was 13; the median score was 1 (0–3). In receiver operating curve analysis, Selvester score ≥1 was identified as the optimum score for predicting presence of LGE enhancement; the area under the curve was 0.826 (95% CI, 0.752–0.900; p < .001). Significant positive correlation was seen between the Selvester score and the extent of LGE enhancement (Spearman ρ, .572; p < .001). The Selvester scoring system correctly identified all LGE segments in 13 of 93 (14%) patients and some LGE segments in 39 (41.9%) patients.
Conclusions
The Selvester QRS score appears to be a convenient and reliable method to determine the presence and extent of myocardial scar in patients with HCM.
Keywords: cardiac magnetic resonance, electrocardiogram, hypertrophic cardiomyopathy, myocardial scar
1.
Hypertrophic cardiomyopathy (HCM), which is characterized by varying degrees of myocardial hypertrophy, is caused by mutations in genes encoding sarcomeric proteins (Liu et al., 2013; Lopes, Rahman, & Elliott, 2013). The clinical course of HCM varies widely (Choi et al., 2010; Kubo et al., 2010), with some patients experiencing only mild symptoms of heart failure late in life and others suffering sudden cardiac death at a young age. Myocardial fibrosis is a hallmark of HCM and is associated with increased risk of arrhythmic events and progression to heart failure. Late gadolinium enhancement (LGE) in contrast‐enhanced cardiovascular magnetic resonance (CMR) has emerged as an in vivo marker of myocardial fibrosis, and the extent of LGE measured by quantitative contrast‐enhanced CMR provides important information for risk stratification among HCM patients (Briasoulis, Mallikethi‐Reddy, Palla, Alesh, & Afonso, 2015; Chan et al., 2014). However, CMR can only be performed at a few large medical centers. A simpler, widely available, and inexpensive method for determining the presence, location, and extent of myocardial fibrosis in HCM patients needs to be found.
In the 1960s, Selvester et al. created an electrocardiogram (ECG)‐based QRS score to measure the extent of scarring in patients with myocardial infarction. This score has since been modified and been found to be useful also in some nonischemic cardiomyopathies, such as dilated cardiomyopathy. The aim of the present study was to determine the value of the Selvester QRS score for identifying the presence, location, and extent of myocardial scar in HCM, using LGE in CMR as the gold standard.
2. METHODS
2.1. Study population
The study population was selected from among the 158 consecutive patients with HCM who were referred to the West China Hospital between January 2012 and July 2016. Patients were eligible for inclusion in this study if two‐dimensional echocardiogram showed left ventricular hypertrophy (wall thickness ≥15 mm) not associated with another cardiac or systemic disease capable of producing hypertrophy of this magnitude. We excluded patients with persistent atrial fibrillation (PAF), coronary heart disease (CHD), primary valvular heart disease, electronic ventricular pacing, prior cardiac surgery, or prior septal ethanol ablation. Patients with renal failure and those who could not undergo CMR scanning because of severe claustrophobia were also excluded.
2.2. CMR protocol
A 3.0T MRI scanner (MAGNETOM Trio Tim; Siemens Medical Solutions) with a dedicated 32‐channel phased‐array cardiac coil was used to perform the CMR imaging. Cardiac cine images were obtained using steady‐state free procession with retrospective electrocardiogram gating during breath‐holds in the three long‐axis planes and the continuous short‐axis planes. The CMR imaging parameters were as follows: field of view, 320–340 mm; flip angle, 50°; echo time, 1.3 ms; matrix size, 256 × 144; repetition time, 3.4 ms; slice thickness, 8 mm with no gap; spatial resolution, 1.4 × 1.3 mm; and temporal resolution, 42 ms. LGE images were acquired 10–15 min after intravenous bolus injection of 0.15 mmol/kg bodyweight of gadopentetate dimeglumine (Magnevist; Bayer Schering Pharma) during breath‐holds and using an inversion recovery turbo fast low‐angle shot sequence with phase‐sensitive reconstruction.
2.3. CMR image analysis
Images were analyzed using dedicated CMR postprocessing software (QMass 8.1; Medis Medical Imaging Systems) by two cardiologists and a radiologist with experience in CMR imaging; all decisions were arrived at by consensus. Ventricular function and mass were assessed according to our previous study. Max LVWT was defined as the maximum thickness of the LV myocardium in all short‐axis slices. The pattern of ventricular septal hypertrophy was classified into the following five morphological subtypes: reverse curvature septum HCM, sigmoid septum HCM, neutral septum HCM, apical HCM, and mid‐ventricular HCM. To quantify the myocardium with LGE, a semi‐automated gray‐scale threshold technique was performed using 6 standard deviations (SD) above the mean of image intensities in a remote normal myocardial region in the same image as generally recommended.
2.4. ECG analysis
All patients underwent 12‐lead electrocardiography. The ECG was recorded with the patient lying supine, article speed of 50 mm/s, and amplification of 20 mm/mV. Analysis of the ECG was performed with the clinician blinded to all clinical and imaging data (other than the patient's age and sex, which are necessary for QRS scoring). The Selvester QRS scoring was performed as described in previous studies. Each score point predicts a scar involving 3% of the total left ventricular (LV) mass. Intraobserver variability was assessed in random samples of 30 ECGs read by the same cardiologists. Interobserver variability was assessed in random samples of 30 ECGs measured by two other cardiologists.
2.5. Statistical analysis
Continuous variables were summarized as the means ± SD, and. compared using the Student's t test (for normally distributed variables) or the Mann‐Whitney U test (for nonnormally distributed variables). Categorical variables were summarized as percentages and compared using the Pearson chi‐square test or the Fisher's exact test. Receiver operating characteristics (ROC) curve analysis was used to assess the value of the Selvester QRS score for predicting myocardial fibrosis. When a significant cutoff value was observed, the sensitivity, specificity, and positive and negative predictive values were presented. Correlation and agreement between the Selvester QRS score and the extent of LGE were examined using the Spearman's correlation coefficient and Bland–Altman analysis, respectively. Statistical analysis was performed using SPSS Statistics, version 21.0 (IBM Corp.). Statistical significance was at p < .05.
3. RESULTS
From among the 158 patients with HCM referred to our center during the study period, 23 patients were excluded: 13 had PAF, six had CHD, and four had undergone prior septal ethanol ablation. Thus, only 135 patients with HCM (69 males, 66 females; mean age, 51 ± 16 years) were included in this study. Table 1 presents the clinical characteristics of the patients.
TABLE 1.
Clinical characteristics of study participants
| Study participants (n = 135) | |
|---|---|
| Male, n (%) | 69 (51) |
| Age, years | 51 ± 16 |
| NYHA functional class | |
| Class I, n (%) | 29 (21) |
| Class II, n (%) | 77 (57) |
| Class III–IV, n (%) | 29 (21) |
| Mean NYHA functional class | 2.0 ± 0.7 |
| NSVT on Holter, n (%) | 25 (19) |
| Resting LVOT gradient | |
| ≥30 mm Hg, n (%) | 76 (56) |
| Hypertension, n (%) | 37 (27) |
| Diabetes, n (%) | 11 (8) |
| Drugs, n (%) | |
| β‐Blockers | 120 (89) |
| Calcium channel antagonists | 16 (12) |
| ACEI/ARB | 18 (13) |
| Diuretics | 25 (19) |
| Amiodarone | 6 (4) |
Abbreviations: ACEI, angiotensin I‐converting enzyme inhibitor; ARB, angiotensin II receptor blockage; LVOT, left ventricular outflow tract; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association.
Table 2 presents the CMR characteristics of the patients. LGE was clearly visualized in 93 of 135 (69%) patients. The mean total LGE burden among the population is 5 (0‐14)g. Among the different morphological subtypes, LGE was most frequently seen in mid‐ventricular HCM (5/5, 100%), followed by reverse curvature septum HCM (43/47, 91%), neutral septum HCM (10/15, 67%), sigmoid septum HCM (29/56, 52%), and apical HCM (6/12, 50%).
TABLE 2.
Cardiac magnetic resonance characteristics of patients with hypertrophic cardiomyopathy
| Study participants (n = 135) | Cases with LGE (n = 93) | Cases without LGE (n = 42) | p Value* | |
|---|---|---|---|---|
| LVEDV, (mL) | 141 ± 36 | 145 ± 37 | 133 ± 30 | .055 |
| LVESV, (mL) | 55 ± 27 | 58 ± 29 | 47 ± 20 | .019 |
| LVEF, (%) | 62 ± 10 | 61 ± 11 | 65 ± 9 | .021 |
| Maximum LV wall thickness, (mm) | 24 ± 6 | 26 ± 6 | 21 ± 3 | <.001 |
| LV mass, (g) | 184 ± 77 | 197 ± 84 | 155 ± 51 | .003 |
| Morphology, n (%) | ||||
| Sigmoid | 56 (41) | 29 (21) | 27 (20) | |
| Reverse | 47 (35) | 43 (32) | 4 (3) | |
| Neutral | 15 (11) | 10 (7) | 5 (4) | <.001 |
| Apical | 12 (9) | 6 (4) | 6 (4) | |
| Mid‐ventricular | 5 (4) | 5 (5) | 0 | |
Abbreviations: LGE, late gadolinium enhancement; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; Max LVT, maximal left ventricular thickness.
p value of comparing between patients with and without LGE myocardial segments
The Selvester QRS score detected fibrosis in 103 of 135 (76%) patients. The scores ranged from 0 to 14 points. One hundred and five (72%) patients had ECG confounders, the most prevalent being left ventricular hypertrophy (90/135, 67%; Table 3). In patients with QRS scores of 0, 1–2, and ≥3, the proportions of those with LGE were 30%, 82%, and 94%, respectively.
TABLE 3.
Electrocardiographic findings in patients with hypertrophic cardiomyopathy
| Study participants (n = 135) | Cases with LGE (n = 93) | Cases without LGE (n = 42) | p Value* | |
|---|---|---|---|---|
| QRS duration (ms) | 100 ± 10 | 102 ± 10 | 99 ± 12 | .792 |
| QTc interval (ms) | 440 ± 35 | 442 ± 35 | 436 ± 32 | .835 |
| ECG characteristics, n (%) | ||||
| LVH | 90 (67) | 61 (45) | 29 (21) | |
| NO confounders | 30 (22) | 20 (15) | 10 (7) | |
| RBBB | 5 (4) | 3 (2) | 2 (1) | .611 |
| LAFB | 6 (4) | 6 (4) | 0 (0) | |
| LBBB | 4 (3) | 3 (2) | 1 (1) | |
| Selvester score, n (%) | ||||
| 0 | 43 (32) | 13 (10) | 30 (20) | |
| 1–2 | 57 (42) | 47 (35) | 10 (10) | |
| 3–4 | 23 (17) | 21 (16) | 2 (1) | <.001 |
| ≥5 | 12 (9) | 12 (9) | 0 (0) | |
Abbreviations: ECG, electrocardiography; LAFB, left anterior fascicular block; LVH, left ventricular hypertrophy; RBBB, left bundle branch block; RBBB, right bundle branch block.
p value of comparing between patients with and without LGE myocardial segments.
The LGE‐positive patients were considerd as patients with myocardial scar. In the ROC analysis, Selvester QRS score ≥1 had 86% sensitivity and 71.4% specificity for prediction of myocardial scar in HCM patients; the area under the curve (AUC) was 0.826 (95% CI: 0.752–0.900; p < .001; Figure 1).
FIGURE 1.
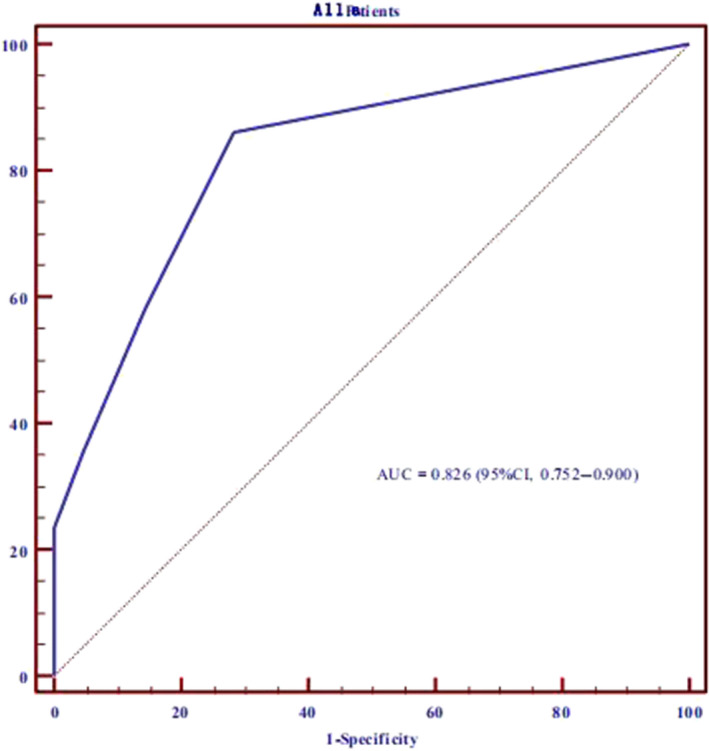
Receiver operating curve analysis shows that Selvester QRS score ≥1 has 86% sensitivity and 71.4% specificity for prediction of presence of myocardial scar in hypertrophic cardiomyopathy (HCM) patients. The area under the curve is 0.826 (p < .001)
Figure 2 shows the Bland–Altman plots for the quantification of myocardial scar by LGE versus Selvester QRS score. The agreement between the two methods was high, with the mean difference being only −1%. There was a significant positive correlation between the Selvester QRS score and the extent of LGE (Spearman's correlation coefficient ρ = .572, p < .001). The correlation was highest in neutral septum HCM (ρ = .765, p = .001) and lowest in reverse curvature septum HCM (ρ = .341, p = .019).
FIGURE 2.
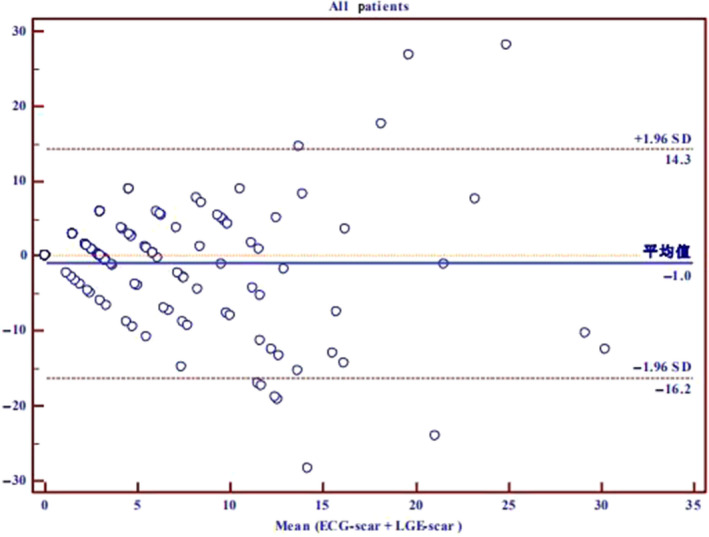
Bland–Altman plots for the quantification of myocardial scar in hypertrophic cardiomyopathy (HCM) patients by LGE versus Selvester QRS score
In the 93 patients with LGE, the Selvester QRS score could completely identify the location of LGE in 13 patients (14%) and partially identify the location of LGE in 39 patients (41.9%).
There were 150 segments where the ECG indicated the presence of myocardial scar although LGE indicated no scarring. The mismatched segments were mainly located in the posterolateral (n = 40), anterior (n = 29), and apical (n = 20) regions.
Interobserver and intraobserver agreement were good for LGE (interobserver ICC = 0.910, p < .001; intraobserver ICC = 0.942, p < .001). For the Selvester QRS score, the intraobserver agreement was good (ICC = 0.898, p < .001), but the interobserver agreement was relatively poor (ICC = 0.770, p < .001).
4. DISCUSSION
This study aimed to investigate the usefulness of the Selvester QRS score for identifying the presence, location, and extent of myocardial scar in HCM. We found that the Selvester QRS score can accurately identify the presence of scar; the score was also found to correlate well with the extent of LGE in CMR.
Hypertrophic cardiomyopathy is a relatively benign disease; in a previous study, the annual mortality rate was only 1.3%. However, this disease is very heterogeneous, and sudden cardiac death and heart failure have also been reported. In fact, HCM is the most common monogenic cardiac disease cause of sudden death in young people (Maron, Rowin, Casey, Garberich, & Maron, 2016). It is therefore important to identify high‐risk patients.
Myocardial fibrosis is a common pathologic feature of HCM. Previous studies have demonstrated that the extent of CMR LGE, which is a measure of myocardial fibrosis, is an independent risk factor for sudden death, heart failure progression, and ventricular arrhythmia in HCM patients (Briasoulis et al., 2015; Chan et al., 2014). However, CMR can only be performed at large centers with modern equipment and trained specialists. The ECG may be a feasible alternative.
Selvester et al. showed that myocardial scar in any part of the left ventricle produced characteristic and quantifiable changes in the ECG. Using the changes in Q‐ and R‐wave durations, R/Q and R/S amplitude ratios, R‐ and S‐wave amplitudes, and R‐wave notches, they developed the QRS score, each score point of which represents infarct involving 3% of the left ventricle (Hindman et al., 1985; Selvester, Kalaba, Collier, Bellman, & Kagiwada, 1967; Wagner et al., 1982). Previous studies have shown that the Selvester QRS score can accurately identify the location of myocardial scar in patients with myocardial infarction and that the score is strongly correlated with the extent of scarring. Moreover, this score was shown to successfully predict the outcomes of myocardial infarction (Horacek et al., 2006; Kurisu et al., 2017; Rosengarten et al., 2013).
The pattern of myocardial fibrosis in nonischemic cardiomyopathy differs from that in ischemic cardiomyopathy. In nonischemic cardiomyopathy, the myocardial fibrosis is usually diffuse and not transmural. It can distribute in subepicardium, subendocardium, and middle myocardium. Strauss et al. (2008) proved that the QRS score can accurately identify and quantify myocardial fibrosis in patients with nonischemic cardiomyopathy; however, their study did not include patients with HCM. Hiraiwa et al. (2018) showed that the Selvester QRS score can predict future cardiac events in patients with dilated cardiomyopathy.
To our knowledge, the present study is the first to prove that the Selvester QRS score can be used to identify the presence and extent of myocardial fibrosis in HCM. However, the correlation that we found between the QRS score and CMR LGE extent (i.e., scar extent) in HCM patients was a little weaker than that reported previously in ischemic and nonischemic cardiomyopathy. We speculate that this difference may be due to the different mechanisms of the abnormal Q wave in HCM. Koga, Yamaga, Hiyamuta, Ikeda, and Toshima (2004) used intracoronary ECG to prove that two different mechanisms exist for the development of abnormal Q waves in patients with HCM: (a) the loss of local electrical forces due to transmural myocardial fibrosis and (b) the altered direction of the initial QRS vector due to the increased electrical forces of the disproportionate hypertrophy of the basal ventricular septum and/or basal left ventricular free wall, unopposed by apical electrical forces. These mechanisms can also explain why correlation between QRS score and CMR LGE extent was highest in neutral septum HCM and lowest in reverse curvature septum HCM.
The current study has some limitations. First, it was an observational study with a small sample. Second, it is unknown how well the CMR LGE can detect and quantify diffuse microscopic nonfocal scarring. An attempt to associate QRS score with the occurrence of ventricular arrhythmias and increased mortality in HCM patients was not within the scope of the current study.
5. CONCLUSION
The Selvester QRS scoring system, which requires only the inexpensive, convenient, and widely available 12‐lead ECG, appears to be a reliable method for determining the presence and extent of myocardial scar in patients with HCM.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Qing Zhang, Shi Chen.Project administration: Xuefeng Wang.Supervision: Yucheng Chen.Validation: Qing Zhang.Writing: Shi Chen, Liwei Huang.
ETHICAL APPROVAL
This study was approved by the Local Ethics Committee of the West China Hospital of SCU.
Chen S, Wang X, Huang L, Chen Y, Zhang Q. Performance of 12‐lead electrocardiogram Selvester QRS scoring criteria to diagnose myocardial scar in patients with hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2020;25:e12762 10.1111/anec.12762
REFERENCES
- Briasoulis, A. , Mallikethi‐Reddy, S. , Palla, M. , Alesh, I. , & Afonso, L. (2015). Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: A meta‐analysis. Heart, 101(17), 1406–1411. 10.1136/heartjnl-2015-307682 [DOI] [PubMed] [Google Scholar]
- Chan, R. H. , Maron, B. J. , Olivotto, I. , Pencina, M. J. , Assenza, G. E. , Haas, T. , … Maron, M. S. (2014). Prognostic value of quantitative contrast‐enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation, 130(6), 484–495. [DOI] [PubMed] [Google Scholar]
- Choi, J.‐O. , Yu, C.‐W. , Chun Nah, J. , Rang Park, J. , Lee, B.‐S. , Jeong Choi, Y. U. , … Euy Park, J. (2010). Long‐term outcome of 4 Korean families with hypertrophic cardiomyopathy caused by 4 different mutations. Clinical Cardiology, 33(7), 430–438. 10.1002/clc.20795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindman, N. B. , Schocken, D. D. , Widmann, M. , Anderson, W. D. , White, R. D. , Leggett, S. , … Wagner, G. S. (1985). Evaluation of a QRS scoring system for estimating myocardial infarct size. V. Specificity and method of application of the complete system. The American Journal of Cardiology, 55, 1485–1490. [DOI] [PubMed] [Google Scholar]
- Hiraiwa, H. , Okumura, T. , Sawamura, A. , Sugiura, Y. , Kondo, T. , Watanabe, N. , … Murohara, T. (2018). The Selvester QRS score as a predictor of cardiac events in nonischemic dilated cardiomyopathy. Journal of Cardiology, 71(3), 284–290. 10.1016/j.jjcc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Horacek, B. M. , Warren, J. W. , Albano, A. , Palmeri, M. A. , Rembert, J. C. , Greenfield Jr., J. C. , & Wagner, G. (2006). Development of an automated Selvester Scoring System for estimating the size of myocardial infarction from the electrocardiogram. Journal of Electrocardiology, 39(2), 162–168. [DOI] [PubMed] [Google Scholar]
- Koga, Y. , Yamaga, A. , Hiyamuta, K. , Ikeda, H. , & Toshima, H. (2004). Mechanisms of abnormal Q waves in hypertrophic cardiomyopathy assessed by intracoronary electrocardiography. Journal of Cardiovascular Electrophysiology, 15(12), 1402–1408. [DOI] [PubMed] [Google Scholar]
- Kubo, T. , Kitaoka, H. , Okawa, M. , Hirota, T. , Hayato, K. , Yamasaki, N. , … Doi, Y. L. (2010). Gender‐specific differences in the clinical features of hypertrophic cardiomyopathy in a community‐based Japanese population: Results from Kochi RYOMA study. Journal of Cardiology, 56(3), 314–319. 10.1016/j.jjcc.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Kurisu, S. , Shimonaga, T. , Ikenaga, H. , Watanabe, N. , Higaki, T. , Ishibashi, K. , … Kihara, Y. (2017). Selvester QRS score and total perfusion deficit calculated by quantitative gated single‐photon emission computed tomography in patients with prior anterior myocardial infarction in the coronary intervention era. Heart and Vessels, 32(4), 369–375. 10.1007/s00380-016-0884-0 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Liu, W. , Hu, D. , Zhu, T. , Ma, Z. , Yang, J. , … Tong, Q. (2013). Mutation spectrum in a large cohort of unrelated Chinese patients with hypertrophic cardiomyopathy. The American Journal of Cardiology, 112(4), 585–589. 10.1016/j.amjcard.2013.04.021 [DOI] [PubMed] [Google Scholar]
- Lopes, L. R. , Rahman, M. S. , & Elliott, P. M. (2013). A systematic review and meta‐analysis of genotype‐phenotype associations in patients with hypertrophic cardiomyopathy caused by sarcomeric protein mutations. Heart, 99(24), 1800–1811. 10.1136/heartjnl-2013-303939 [DOI] [PubMed] [Google Scholar]
- Maron, B. J. , Rowin, E. J. , Casey, S. A. , Garberich, R. F. , & Maron, M. S. (2016). What do patients with hypertrophic cardiomyopathy die from? The American Journal of Cardiology, 117(3), 434–435. 10.1016/j.amjcard.2015.11.013 [DOI] [PubMed] [Google Scholar]
- Rosengarten, J. A. , Scott, P. A. , Chiu, O. K. , Shambrook, J. S. , Curzen, N. P. , & Morgan, J. M. (2013). Can QRS scoring predict left ventricular scar and clinical outcomes? Europace, 15(7), 1034–1041. 10.1093/europace/eut014 [DOI] [PubMed] [Google Scholar]
- Selvester, R. H. , Kalaba, R. , Collier, C. R. , Bellman, R. , & Kagiwada, H. (1967). A digital computer model of the vectorcardiogram with distance and boundary effects: Simulated myocardial infarction. American Heart Journal, 74, 792–808. 10.1016/0002-8703(67)90098-1 [DOI] [PubMed] [Google Scholar]
- Strauss, D. G. , Selvester, R. H. , Lima, J. A. C. , Arheden, Håkan , Miller, J. M. , Gerstenblith, G. , … Wu, K. C. (2008). ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: Correlation with cardiac magnetic resonance and arrhythmogenesis. Circulation: Arrhythmia and Electrophysiology, 1(5), 327–336. 10.1161/CIRCEP.108.798660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, G. S. , Freye, C. J. , Palmeri, S. T. , Roark, S. F. , Stack, N. C. , Ideker, R. E. , … Selvester, R. H. (1982). Evaluation of a QRS scoring system for estimating myocardial infarct size. I. Specificity and Observer Agreement. Circulation, 65, 342–347. [DOI] [PubMed] [Google Scholar]


