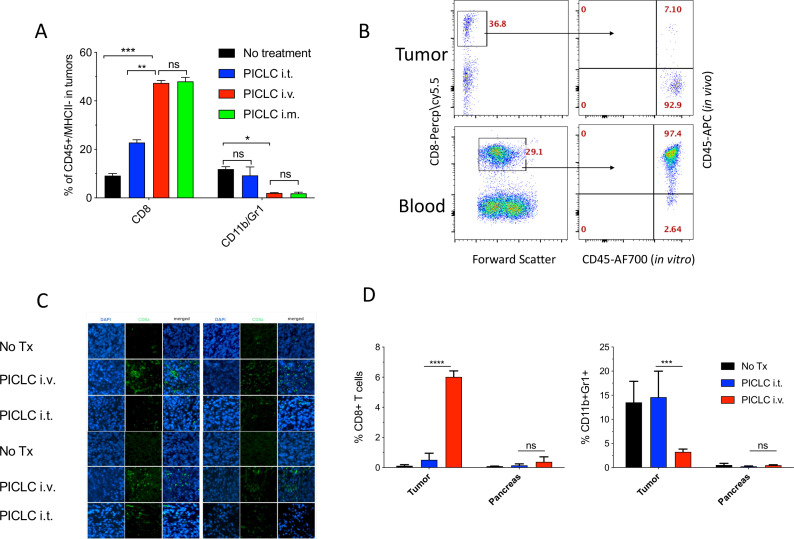
Figure 2.
Systemic poly-IC stabilized with poly-lysine and carboxymethylcellulose (PICLC) elicits CD8 T cell infiltrates into the tumor parenchyma. (A) CD8+ T cell and myeloid-derived suppressor cell (MDSC) infiltration in B16F10 tumors after treatments. Wild-type (WT) mice were inoculated subcutaneously on day 0 with 3×105 B16F10 cells and injected intravenously, intramusculary or intratumorally with 50 µg PICLC on days 7, 12 and 17. On day 19, 5 min prior to tumor harvests the mice received 5 µg anti-CD45-APC intravenously and the % of CD8+ T and CD11b+Gr1+ cells were measured in the tumor single cell suspension. Bars are shown as mean±SD; n=3 mice per group. *P<0.05, **p<0.01 and ns, not significant by Mann-Whitney unpaired test. (B) In vivo CD45 staining as described in ‘Materials and methods’ section. A representative dot plot showing that the majority of the CD8 T cells harvested from tumor of an intravenous PICLC-treated mouse did not stain with the in vivo administered anti-CD45-APC, while most of the blood CD8 T cells did. (C) Immunofluorescence staining for the tumor tissue after PICLC treatment. Similar experiment to (A) except tumors were fixed in 2% formalin and stained with anti-CD8α monoclonal antibody (mAb) and DAPI and observed by fluorescence microscopy. (D) CD8+ T cells and MDSCs infiltration in tumors and the pancreas after PICLC treatment. WT mice were inoculated subcutaneously on day 0 with 5×105 Lewis lung carcinoma (LLC) cells and injected intravenously or intratumorally with 50 µg PICLC on days 9, 14 and 19, and the percentages of CD8+ T and CD11b+Gr1+ cells of all the live cells were measured in tumor and pancreas single cell suspensions 24 hours after the last PICLC injection. Bars are shown as mean±SD; n=2–3 mice per group. ****P<0.0001 ***p<0.001 **p<0.01 and ns, not significant by Mann-Whitney unpaired test.