Fig. 15.5.
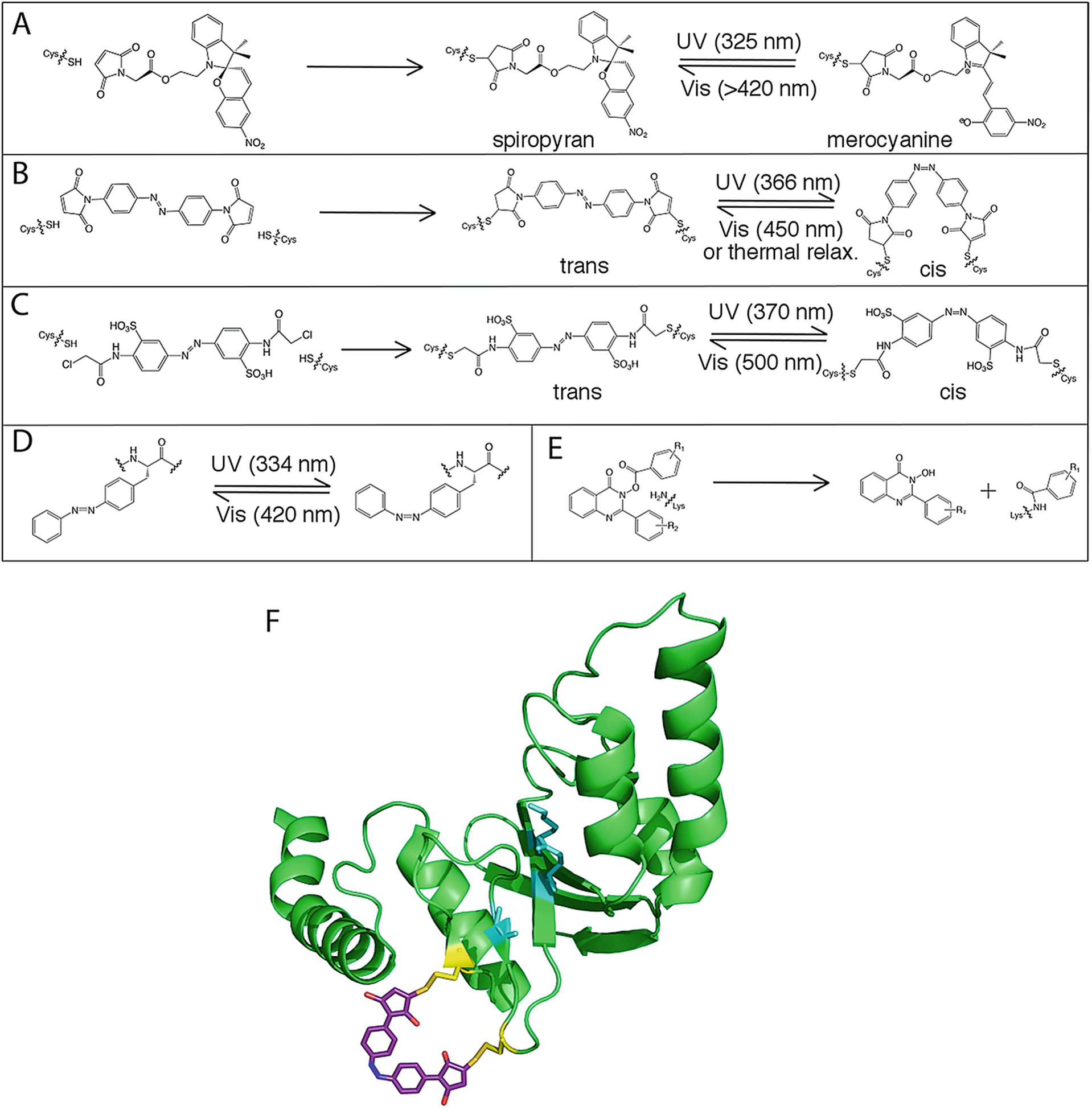
Covalent modification of proteins to control allostery. (a) Conjugation of spiropyran to a Cys residue and isomerization to the merocyanine form. (b) Conjugation of the bifunctional azobenzene derivative 4,4′-bis (maleimido)azobenzene to two cysteine residues and isomerization between trans and cis forms. (c) Conjugation of the bifunctional azobenzene derivative 3,3′-bis (sulfonato)-4,4′-bis (chloroacetamido)azobenzene (BSBCA) to two Cys residues and isomerization between trans and cis forms. (d) Isomerization of the genetically encoded azobenzene derivative AzoPhe. (e) Covalent modification of a Lys residue by quinazolin-4 (3H)-one hydroxamic ester. (f) Covalent modification of PvuII with azomal (PDB: 1NI0), adapted from Schierling et al. [108]. Active site residues (cyan) are disrupted upon isomerization of azomal to the trans form
