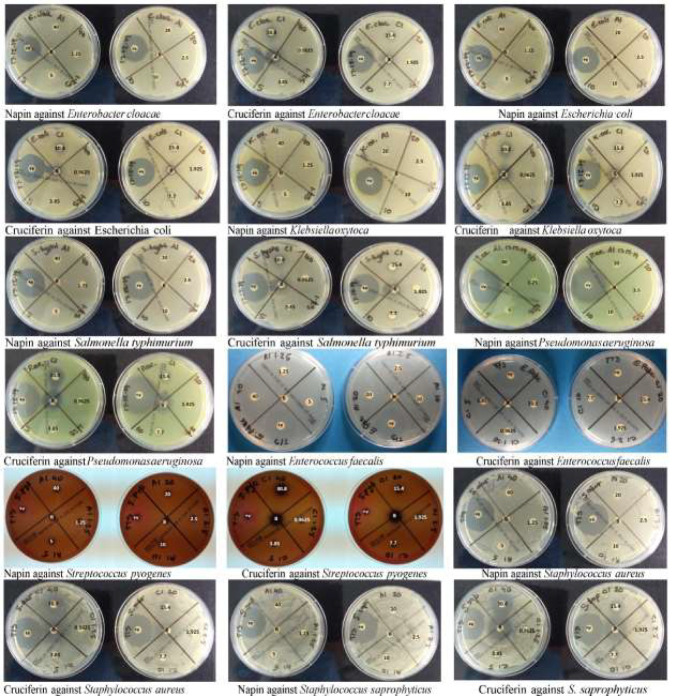
Figure 4.
Plate images of disc diffusion antimicrobial susceptibility testing as per EUCAST guidelines for napin and cruciferin proteins against four Gram positive and five Gram negative bacteria, labeled electronically to reflect the actual concentrations tested. The agar plates used for the testing of the purified napin protein are labeled with the doses corresponding to 40 µg down to 1.25 µg, while plates used to test the purified cruciferin protein are labeled with the doses corresponding to 30.8 µg down to 0.9625 µg. The plates were labeled prior to the solubilization of the proteins (and subsequently, lower working concentrations were prepared for cruciferin 1, due to additional solvents added for solubilization). +v indicates that positive control antibiotics showing the expected zones of inhibition ≥14 mm (1.25 µg/23.75µg of Trimethoprim/Sulfamethoxazole) and ≥26 mm (5-µg Ciprofloxacin). B at the center indicates the blank negative control.