Abstract
Purpose:
To analyze the overall clinical outcomes of Descemet membrane endothelial keratoplasty (DMEK) in 600 consecutive cases.
Methods:
Retrospective, consecutive interventional case series operated by a single surgeon. Six hundred consecutive eyes of 524 patients with endothelial dysfunctions of different etiologies scheduled for DMEK were included in this study. All donor tissues were prepared by the operating surgeon during the procedure, using McCarey Kaufman medium or Cornisol-preserved cornea with endothelial cell density (ECD) of ≥2500 cells/mm2. Indications, postoperative best spectacle-corrected visual acuity (BSCVA), ECD, endothelial cell loss (ECL), and complications were analyzed postoperatively between 3 months and 2 years.
Results:
The commonest indication was post-cataract corneal edema/bullous keratopathy in 262 (43.7%) eyes followed by Fuchs′ endothelial corneal dystrophy 218 (36.3%). Vision affected comorbidities were present in 91 (15.2%) eyes. In phakic eyes with cataract (222; 37%), DMEK was combined with cataract surgery (Triple-DMEK). BSCVA of ≥20/25 was achieved in 41.0%, 46.4%, 49.2%, and 48.7% of eyes at 3, 6, 12, and 24 months, respectively and stabilized at 6 months (P = 0.54). Mean ECD decreased from 2884 ± 178 cells/mm2 (n = 600) before surgery to 2223 ± 321 (n = 597), 2099 ± 354 (n = 524), 1918 ± 373 (n = 374), and 1772 ± 439 cells/mm2 (n = 158) at 3, 6, 12, and 24 months respectively. The corresponding mean ECL was 22.9 ± 11.4%, 27.2 ± 12.4%, 33.5 ± 13.0%, and 38.6 ± 14.3%, respectively (P < 0.05 for all-time points). The commonest complication was DM detachment in 59 (9.8%) eyes of which 23 (3.8%) eyes required rebubbling. Three (0.5%) eyes had primary graft failure. Endothelial rejection occurred in 7 (1.2%) eyes until the last follow-up.
Conclusion:
DMEK is a safe and effective procedure in different types of endothelial diseases with encouraging surgical and clinical outcomes. Complications are less and ECL percentage up to 2 years is acceptable.
Keywords: Descemet membrane endothelial keratoplasty, endothelial cell density, endothelial cell loss, surgeon’s prepared donor tissue, visual outcomes
For last two decades, endothelial keratoplasty (EK) has become a well-established surgical procedure for corneal endothelial diseases/dysfunctions such as post-cataract corneal edema or bullous keratopathy (PCE/PBK), Fuchs’ endothelial corneal dystrophy (FECD), previous failed penetrating keratoplasty, or Descemet stripping (automated) endothelial keratoplasty (DSEK/DSAEK) and the others. DSEK/DSAEK is the most common type of EK procedure across the world.[1] However, DSEK/DSAEK is not the true anatomic replacement surgery of the diseased innermost corneal layers. The stroma-to-stroma interface irregularities with more hyperopic shift cause delayed visual rehabilitation, and also with higher-order aberrations and less than perfect visual outcomes.[2]
In 2006, Melles et al. performed the first Descemet membrane-endothelium (DM-E) complex transplant which is a true anatomical replacement surgery, and he named it Descemet membrane endothelial keratoplasty or DMEK.[3] After 10 years of that procedure, the same patient’s vision was stable at 20/20 in both eyes with an endothelial cell loss (ECL) of 72% and 68% without any rejection episode.[4] Over the last few years, this procedure has shown potentially better visual outcomes and faster recovery than DSEK/DSAEK, though the initial learning curve is very much steeper, and there is a chance of more ECL due to more donor manipulation during the surgery.[5] However, with time and more experiences, DMEK has been evolved as a standardized, “no-touch” technique, with better results in terms of visual outcomes and ECL.[6,7,8] The statistical report from Eye Bank Association of America shows that in 2018; of all EK procedures, DSAEK/DSEK was performed 19,526 times (a decrease of 5.8%) while DMEK was performed 10,773 times (an increase of 6.2%) compared to year 2017, in the USA.[9]
In the Indian context, the overall scenario of DMEK procedure for endothelial disorders is different and may be difficult. Unlike the western patient population, Indian patients requiring EK, present late in the clinic with advanced endothelial diseases, and most of these diseased eyes are with best spectacle-corrected visual acuity (BSCVA) of ≤20/200.[10] Moreover, DMEK donor tissue (DM-scroll) unscrolling becomes difficult due to poor view in presence of dark or deep brown iris background in Indian eyes, than against blue or light-colored iris of eyes in western population.[11,12] Again, unlike eye banks in the western world, Indian eye banks do not supply pre-stripped, prestamped, prestained, or preloaded donor DM-scroll as readymade donor tissue for DMEK procedure to the corneal surgeons.[13,14] Most of the Indian eye banks usually preserve the corneoscleral (CS) button either in McCarey Kaufman medium (MKM) (Ramayamma International Eye Bank, LV Prasad Eye Hospital, Hyderabad, India) or in Cornisol medium (CSM) (AuroLab, Madurai, India).[15] To date, there are two studies from India about outcomes of DMEK in Indian eyes where the surgeons used only CSM-preserved donor cornea.[11,12] In another study from India, the authors have published their short-term results of DMEK in consecutive first 100 eyes using surgeon’s prepared donor corneas with good clinical outcomes and acceptable ECL after 3 months.[16]
The purpose of the present study was to report the indications, clinical outcomes, endothelial cell density (ECD), and complications following consecutive 600 DMEK procedures over the last 3 years at a tertiary referral eye hospital.
Methods
This was a retrospective, noncomparative consecutive interventional large case series of all eyes that underwent DMEK performed between 1st April 2016 and 30th April 2019 by a single surgeon. Informed written consent was taken from all patients prior to the surgical procedure. The study was approved by the institutional ethics committee, and the study was conducted according to the Declaration of Helsinki. Six hundred consecutive eyes of 524 patients with endothelial diseases/dysfunctions of different etiologies, such as PCE/PBK, FECD, previous failed PK or DSEK/DSAEK, and the others were included in this study. Preoperatively, all patients underwent BSCVA testing using the Snellen chart, slit-lamp evaluation, lens status, intraocular pressure (IOP) measurement and dilated fundus examination if possible. Ultrasonography (USG) B-scan was done in those eyes where the fundus details were not clearly visible. In selective eyes, anterior segment optical coherence tomography (AS-OCT) (OPTOVUE Inc. Fremont, CA, USA) was done preoperatively to assess epithelial hypertrophy or stromal scarring. In some eyes if possible, macular OCT was also done prior to surgery with the same OCT machine.
The exclusion criteria included severe corneal edema with extremely poor visibility, associated significant stromal scarring, extensive peripheral anterior synechia, and uncontrolled end-stage glaucoma, aphakia with grossly distorted pupil, aniridia, and significant posterior segment pathology as detected by USG B-scan.
Donor cornea
The donor CS buttons, preserved either in MKM or CSM were provided by the institutional eye bank, a nonprofit organization situated within the hospital premises. Excellent grade optical quality donor tissue between 37 and 94 years of age with an endothelial cell count ≥ 2500/mm2 was used. The baseline donor central ECD was measured by an eye bank specular microscope (KeratoAnalyzer – EKA-10, Konan Medical Inc., Hyogo, Japan). The eye bank also provided additional stand-by donor cornea for initial 50 cases.
DMEK graft preparation
DMEK donor graft was prepared by the operating surgeon in the operating room just prior to donor insertion during the surgical procedure. DMEK graft preparation has already been described previously.[16] A 7.00 to 8.50 mm DM-graft was used for transplantation depending upon the case and recipient’s corneal diameter. Trypan blue stained DM-scroll was temporarily transferred to a small petri dish, containing a balanced salt solution (BSS) before loading into an injector system made from IOL cartridge before donor insertion. DM-scroll preparation time (in min) was calculated by a second observer after watching all the video records right from the placement of donor CS button on the Teflon block to shifting of the DM-scroll into the BSS-filled petri dish.
Surgical technique
All surgeries were performed under peribulbar or sub-Tenon anesthesia, except in children where it was performed under general anesthesia. For DMEK-alone cases, the pupil was constricted by instillation of 2% pilocarpine eye drops three times 30 min prior to surgery. Pupillary dilation was required in all phakic eyes where DMEK was combined with cataract surgery with intraocular lens implantation (Triple-DMEK). The details of the surgical procedure for DMEK-alone or Triple-DMEK has also been described earlier.[16] In some patients who required epithelial debridement, a bandage contact lens (BCL) was placed at the end of the procedure.
After the surgery, the patient was shifted to the recovery room and was asked to maintain a supine position for at least 1 hour. After 1 to 1.5 hour, the patient was examined under slit-lamp to check the fluid level in the anterior chamber was above the inferior peripheral iridectomy (PI) or not. If the fluid level was not visible, little burping of air was done via one of the side-ports, and instantaneously fluid level was visible above the PI. As a hospital policy, all patients were kept overnight in the hospital and instructed to maintain supine position. The patients were discharged the next morning after slit-lamp examination.
Postoperative medication and follow-up
Postoperatively, all patients received moxifloxacin (0.5%) eye drops, 4 times daily for 7 days and prednisolone (1.0%) eye drops 6 times daily for 7 days. On day 7, topical prednisolone was reduced to 4 times for 3 months and then gradually weaned over for the next 6 months to once-daily maintenance dose till 1 year. After 1 year, prednisolone eye drop was replaced by fluorometholone (0.1%) eye drop once-daily dose and the patient was asked to continue it lifelong. Temporary BCL was removed after 7 days. Patients were followed up on day 7, 1 month, 3 months, 6 months, and then yearly; or more frequently if necessary.
During each visit, BSCVA was measured using the Snellen chart. A detailed slit-lamp examination was performed to check the graft transparency and IOP was measured. AS-OCT was done in some eyes with suspected DM detachment on day 7 or if required later on. [Fig. 1a and 1b] Similarly, macular OCT was done in selective cases with suboptimal visual outcomes. Postoperative ECD was measured with the clinical specular microscope (EM 3000; Tomey, Nagoya, Japan) for all patients after 3 months, 6 months, and then yearly by an experienced technician. An average of three counts from the central cornea with counting at least 100 cells were considered for this study.
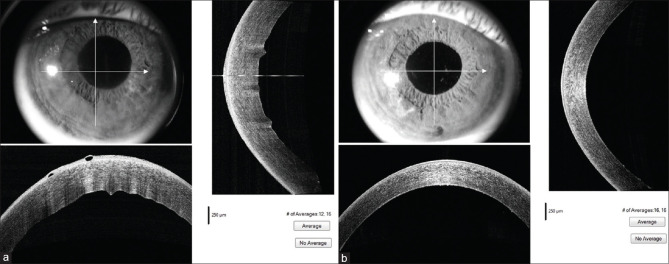
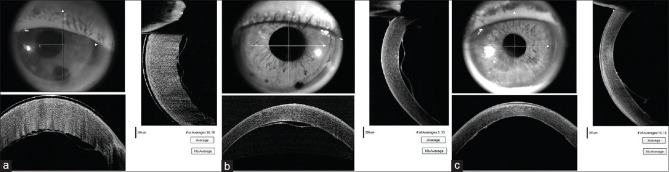
Figure 1.
(a) Preoperative ASOCT image in pseudophakic bullous keratopathy. (b) Postoperative ASOCT image of the same eye 3 weeks after DMEK. [ASOCT – Anterior segment optical coherence tomography]
Statistical analysis
The data was collected and tabulated using Microsoft Excel (Office 2017, Microsoft Corp, Redmond, Washington, USA) and analyzed using RStudio (ver 1.2, RStudio Inc). Snellen BSCVA was converted to logarithms of the minimum angle of resolution (LogMAR) for statistical analysis and graphical representation.[17] The results of continuous measurements were presented as mean ± standard deviation (mean ± SD) and the range; and the results of categorical measurements were presented as number (percentage, %). The statistical significance of BSCVA at various time points was calculated using Mann-Whitney U test. A P value of < 0.05 was considered statistically significant.
Results
Patients’ demographics
Six hundred eyes of 524 patients with endothelial dysfunctions were included in this study. The mean age of the recipient was 62.2 ± 11.6 years. The authors have previously published short-term outcomes of the first 100 DMEK cases and those patients were included in this study with updated follow-up data.[16] A most common indication for DMEK in this series was PBK/PCE in 43.5%, followed by FECD in 36.3%. Preoperatively, the BSCVA in the affected eye was between light perception (LP) and < 20/200 in 343 (57.2%) eyes. In 222 (37%) eyes, DMEK was combined with cataract surgery (Triple-DMEK). Vision affected comorbidities were present in 91 (15.2%) eyes. The median follow-up of this series was 17 months (range: 3 months to 3 years). The number of patients who had completed follow-up at 3 months, 6 months, 12 months, and 24 months were 597, 524, 374, and 158, respectively. Patients lost to follow-up or with missed follow-ups were: at 3 months, 3 (0.5%) eyes (597/600); at 6 months, 5 (0.9%) eyes (524/529); at 1 year, 11 (2.9%) eyes (374/385), and at 2 years, 13 (7.6%) eyes (158/171). All data till last available follow-up was used for statistical analysis. We did not consider those missing data for that time point for calculations. Patients’ demographics and clinical presentation details are shown in Table 1.
Table 1.
Patients’ profile and preoperative ocular status (n=600; 524 patients)
| Age (year; mean and range) | 62.2±11.6 (3-86) | Percentage |
|---|---|---|
| Gender | ||
| Male | 239 | 45.6% |
| Female | 285 | 54.4% |
| Preoperative BSCVA | ||
| LP-<20/200 | 343 | 57.2% |
| 20/200-<20/60 | 218 | 36.3% |
| 20/60-20/30 | 39 | 6.5% |
| ≥20/25 | 0 | 0 |
| Indications | ||
| PCE/PBK | 261 | 43.5% |
| FECD | 218 | 36.3% |
| Post DSEK failed graft | 27 | 4.5% |
| HSV endotheliitis-induced corneal edema | 21 | 3.5% |
| ICE syndrome | 21 | 3.5% |
| PPCD | 14 | 2.3% |
| Post PK failed graft | 12 | 2.0% |
| Post-trabeculectomy or Post-tube corneal edema | 10 | 1.7% |
| Failed DMEK graft (Re-DMEK; PGF-3 and Late failure -4) | 7 | 1.2% |
| Miscellaneous (Post YAG PI-4 CHED-2; ABK-2; CMV-E-1) | 9 | 1.5% |
| Lens status | ||
| Pseudophakic | 372 | 62% |
| Phakic | 226 | 37.7% |
| Aphakic | 2 | 0.3% |
| Comorbidity (with one or more comorbidities) | ||
| Total eyes | 91 | 15.2% |
| Prior Glaucoma with or without surgery | 47 | 7.8% |
| Post VR surgery (with or without SF IOL) | 12 | 2.0% |
| Toxic anterior segment syndrome (TASS) | 16 | 2.7% |
| Vitreous in the anterior chamber | 17 | 2.8% |
| Cystoid macular edema | 17 | 2.8% |
| Other known retinal pathology | 13 | 2.2% |
BSCVA – best-corrected visual acuity; LP – light perception; PCE/PBK – post-cataract corneal edema or bullous keratopathy; FECD – Fuchs’ endothelial corneal dystrophy; DSEK – Descemet stripping endothelial keratoplasty; HSV – herpes simplex virus; ICE – iridocorneal endothelial; PPCD – posterior polymorphous corneal dystrophy; PK – penetrating keratoplasty; PGF – primary graft failure; YAG – yttrium aluminum garnet; CHED – congenital hereditary endothelial dystrophy; ABK – aphakic bullous keratopathy; CMV-E – cytomegalovirus endotheliitis; VR – vitreoretinal; SF IOL – scleral fixation intraocular lens
Donor demographics
Around 611 donor corneas were used in this series for 600 DMEK procedures. The mean donor age was 65.7 ± 10.4 years (range: 37–94 years) and mean central ECD was 2884 ± 178 cells/mm2 (range: 2504–3636 cells/mm2). The mean DM-scroll preparation time was 7.5 ± 2.9 min (range: 3.5–17 min) considering all age groups and both media. The most common DMEK-graft size used was 8.0 mm (63%). Donor characteristics, DM peeling, and DM-scroll preparation time between MKM- and CSM-preserved cornea were not statistically significant (P = 0.54) [Table 2]. About 102 (16.7%) donor tissues were paired (from 51 donors) and they behaved in similar ways during donor preparation. In 5 (0.9%) donor eyes, the DM-E complex was damaged during DM-graft preparation. In 3 cases, donor unscrolling was not possible because of fibrinous reaction in the recipient’s AC and eventually damaged. They were immediately changed with new donor DM-scrolls and surgery completed. In two eyes, DM-scroll slipped out of the AC and got damaged. [Fig. 2] In one eye, DM-graft unscrolling could not be completed, because of visibility problem.
Table 2.
Donor profile and DMEK-graft profile (n=611§,#) (Paired tissue - 102)
| MKM preserved cornea | CSM preserved cornea | P | |
|---|---|---|---|
| Age of donor (year; mean; range) | 65.2±10.7 (40-94) | 66.1±9.6 (37-92) | 0.66 |
| Gender of donor: Male : Female | 167 : 121 | 179 : 144 | 0.55 |
| Total number of donor cornea (age-wise) | 288+2* | 318+3* | 0.60 |
| <55 years (%) | 32 | 34 | |
| 55-75 years (%) | 197 | 219 | |
| >75 years (%) | 61 | 68 | |
| Death to media time (min; mean; range) | 251±65 (110-325) | 247±69 (115-350) | 0.16 |
| Media to surgery time (h: mean; range) | 40.2±11.5 (32-72) | 54.2±26.6 (36-110) | <0.05 |
| Donor ECD (cell/mm2; mean; range) | 2894±169 (2504-3484) | 2874±190 (2521-3636) | 0.12 |
| DM-roll preparation time (min: mean; range) | 7.4±2.4 (3.0-14) | 7.6±3.4 (3.5-17) | 0.54 |
| DMEK-graft size used | Number | Percentage | |
| 7.00 mm | 16 | 2.7% | |
| 7.25 mm | 26 | 4.3% | |
| 7.50.mm | 53 | 8.8% | |
| 7.75 mm | 64 | 10.7% | |
| 8.00 mm | 381 | 63.5% | |
| 8.25 mm | 52 | 8.7% | |
| 8.50 mm | 8 | 1.3% | |
MKM – McCarey Kaufman medium; CSM – Cornisol medium; DM – Descemet membrane; ECD – endothelial cell density; §Overall tissue exchanged – 11 eyes (1.8%), #6 tissues – damaged during unscrolling; *5 tissues – damaged during DM peeling
Figure 2.
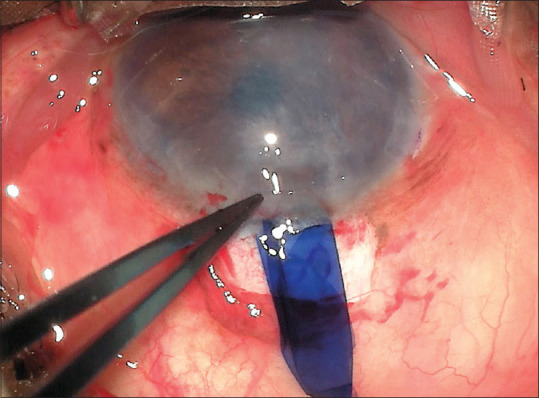
DMEK-scroll slipped out of the anterior chamber and got damaged. [DMEK – Descemet membrane endothelial keratoplasty]
Visual outcomes
The overall visual outcomes in this large DMEK series were highly satisfying irrespective of the indication [Figs. 3a, b; 4a, b; 5a, b and 6a, b]. The mean BSCVA from preoperative levels improved significantly in the postoperative period (P < 0.0001) and vision stabilized after 3 months (P = 0.94 at all-time points) [Fig. 7]. After 3 months, 41.0% of eyes achieved a BSCVA of ≥20/25 (n = 597), and after 2 years 48.7% maintained BSCVA of ≥20/25 (n = 158), which did not differ from the outcome after 1 year with 49.2% of eyes achieving a BSCVA of ≥20/25 (n = 374) (P = 0.76) [Fig. 8].
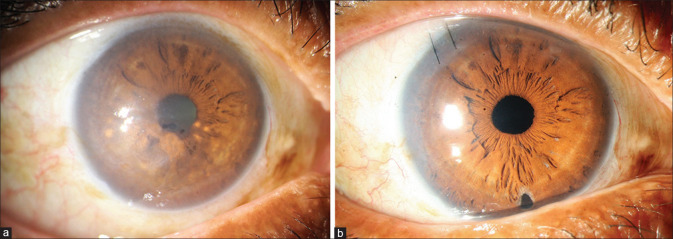
Figure 3.
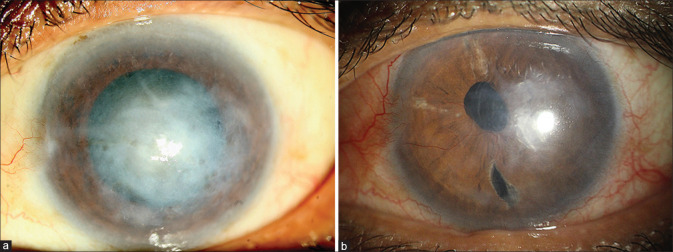
Fuchs’ endothelial corneal dystrophy with corneal edema and cataract. (a) Preoperative. (b) Postoperative: 3 weeks after Triple-DMEK. [Triple-DMEK – Descemet membrane endothelial keratoplasty and cataract surgery with IOL implantation]
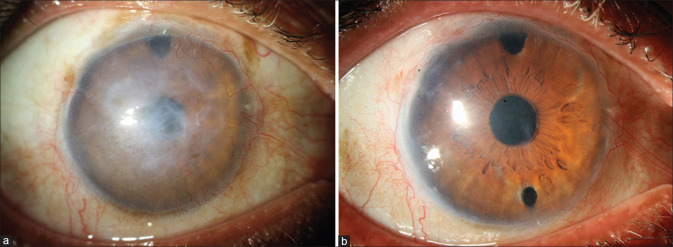
Figure 4.
Pseudophakic bullous keratopathy. (a) Preoperative. (b) Postoperative: same eye 4 weeks after DMEK. [DMEK – Descemet membrane endothelial keratoplasty]
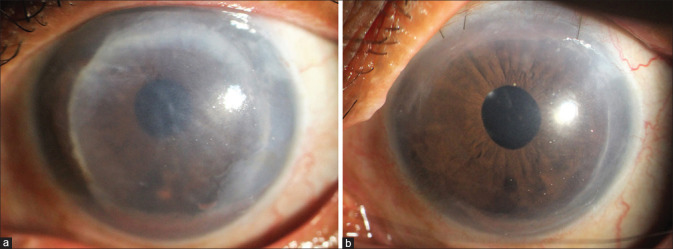
Figure 5.
Failed Descemet stripping endothelial keratoplasty (performed 7 years back). (a) Preoperative. (b) Postoperative: 4 weeks after DMEK. [DMEK – Descemet membrane endothelial keratoplasty]
Figure 6.
HSV-induced severe corneal edema with paracentral stromal scarring. 64 years-male, one-eyed farmer. (a) Preoperative appearance. (b) Postoperative 3 months after Triple-DMEK. Paracentral residual scarring present. BSCVA: 20/40. [HSV - Herpes simplex virus endothelitiis; Triple - DMEK – Descemet membrane endothelial keratoplasty with cataract surgery and IOL implantation; BSCVA – best spectacle-corrected visual acuity]
Figure 7.
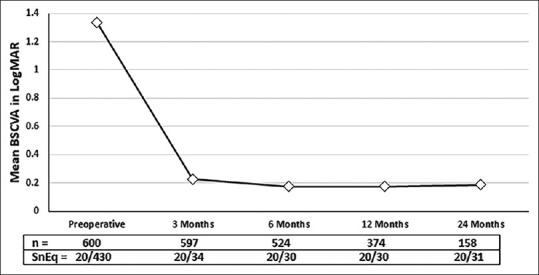
Mean BSCVA: Preoperative and postoperative at 3 months, 6 months, 12 months and 24 months. [BSCVA – best spectacle-corrected visual acuity; LogMAR - logarithms of the minimum angle of resolution; SnEq – Snellen’s equivalent; n – number of eyes]
Figure 8.
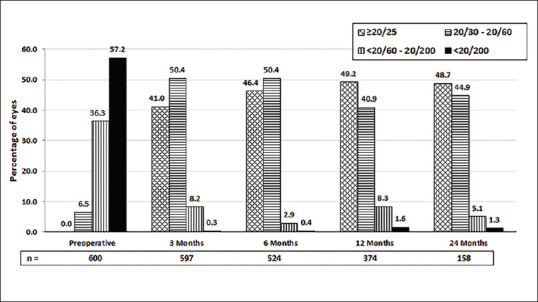
Percentage of eyes with best spectacle-corrected visual acuity in Snellen’s chart: preoperative and postoperative periods. [n – number of eyes]
Endothelial cell density
Mean ECD decreased from 2884 ± 178 cells/mm2 (n = 600) before surgery to 2223 ± 321 (n = 597), 2099 ± 354 (n = 524), 1918 ± 373 (n = 374), and 1772 ± 439 cells/mm2 (n = 158) at 3, 6, 12, and 24 months, respectively. The corresponding mean ECL was 22.9 ± 11.4%, 27.2 ± 12.4%, 33.5 ± 13.0%, and 38.6 ± 14.3%, respectively [Fig. 9]. Postoperatively, the ECD decrease slowed down considerably after 3 months. The details of the operative results are shown in Table 3.
Figure 9.
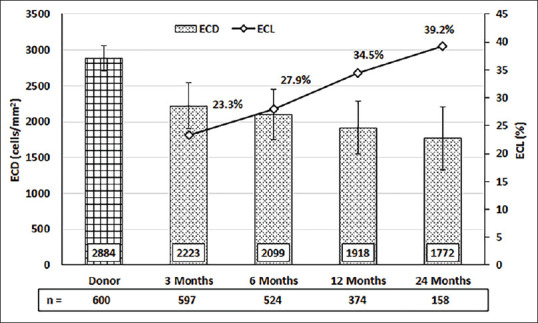
Postoperative ECD and ECL at various time points. The bar graph and corresponding left y-axis show average ECD and error bars showing the standard deviation. The line graph and corresponding right y-axis show the ECL% change. [ECD - endothelial cell density; ECL – endothelial cell loss; n – number of eyes]
Table 3.
Overall Outcomes: BSCVA, Endothelial Cell Density, and Endothelial Cell Loss
| Clinical outcomes | Preoperative (n=600) |
At 3 Months (n=597) |
At 6 Months (n=524) |
At 12 Months (n=374) |
At 24 Months (n=158) |
|---|---|---|---|---|---|
| BSCVA | n (%) | n (%) | n (%) | n (%) | (n; %) |
| ≥20/25 | 0 (0%) | 245 (46.9%) | 243 (48.0%) | 184 (49.2%) | 75 (48.7%) |
| 20/30-20/60 | 39 (6.5%) | 301 (42.7%) | 264 (40.2%) | 153 (40.9%) | 69 (44.9%) |
| <20/60-20/200 | 218 (36.3%) | 49 (8.6%) | 15 (2.9%) | 31 (8.3%) | 8 (5.1%) |
| <20/200 | 343 (57.2%) | 2 (0.3%) | 2 (0.4%) | 6 (1.6%) | 2 (1.3%) |
| ECD (mean±SD); cells/mm2 | 2884±178 | 2223±321 | 2099±354 | 1918±373 | 1772±439 |
| ECL (mean±SD) % | 22.9±11.4% | 27.2±12.4% | 33.5±13.0% | 38.6±14.3% |
BSCVA – best spectacle-corrected visual acuity; ECD – endothelial cell density; ECL – endothelial cell loss
Complications
The commonest complications observed in this series was DM detachment in various form in 59 (9.8%) eyes within the first month [Fig. 10a–c]. In 23 (3.8%) eyes, the DM detachment was > 1/3rd of the graft surface area and required rebubbling with air. Six eyes required repeat rebubbling. In 34 (5.6%) eyes with peripheral DM detachment (<1/3rd area) or with small central/paracentral detachment, DM-graft attached spontaneously with time. Two eyes had total graft detachment (floating DM-scroll) which required retransplantation. Iatrogenic primary graft failure (PGF) happened in 3 (0.5%) eyes and re-DMEK were done in those cases after 2–4 weeks. Other graft-related complications up to 3 years after DMEK were secondary graft failure (SGF) in 6 (1.0%) eyes and endothelial allograft rejection in 7 (1.2%). Five of the rejection episodes could be reverted by medical management. [Fig. 11a–f] but 2 of them required re-grafting. Within this study period, a total of 13 eyes (2.2%) required re-transplantation of which re-DMEK was performed in 7 (1.2%) eyes, secondary DSEK/DSAEK in 5 eyes, and penetrating keratoplasty in 3 eyes. The details of all complications are listed in Table 4.
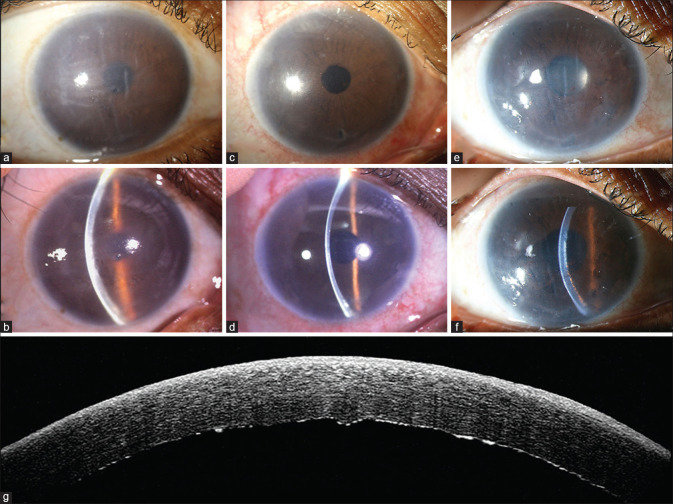
Figure 10.
ASOCT image showing various types of DM detachment. (a) Total DM detachment - required rebubbling. (b) Partial central DM detachment - resolved spontaneously within 3 weeks. (c) Partial peripheral DM detachment. resolved spontaneously within 4 weeks. [ASOCT – Anterior segment optical coherence tomography; DM – Descemet membrane]
Figure 11.
DMEK graft rejection. (a and b) Preoperative; (c and d) Clear graft after 3 months. (e and f) Graft rejection at 11 months. Slit image shows multiple KPs with corneal edema. (g) ASOCT image of DMEK-graft rejection which shows the KPs more clearly. [DMEK – Descemet membrane endothelial keratoplasty; ASOCT – Anterior segment optical coherence tomography; KP – Keratic precipitates]
Table 4.
Overall surgical and postoperative complications (n=600)
| Complications | Number | Percentage |
|---|---|---|
| Operative | ||
| Graft damage during the preparation | 5 | 0.8% |
| Bleeding from the PI site | 37 | 6.2% |
| Excessive fibrinous reaction (in TASS cases) | 6 | 1.0% |
| Unable to unscroll DM-graft (due to entangled fibrin thread - 3; extremely poor visibility - 1) | 4 | 0.7% |
| Donor out of the anterior chamber | 2 | 0.3% |
| DM-Graft behind the iris | 1 | 0.2% |
| Postoperative | ||
| Pupillary block | 3 | 0.5% |
| Detachment of DM graft (Total) | 59 | 9.8% |
| Complete detachment | 2 | 0.3% |
| Partial central/paracentral | 7 | 1.2% |
| <1/3rd | 5 | 0.8% |
| >1/3rd | 2 | 0.3% |
| Peripheral detachment | 50 | 8.3% |
| <1/3rd | 29 | 4.8% |
| >1/3rd | 21 | 3.5% |
| Steroid-induced glaucoma | 43 | 7.1% |
| Primary graft failure (PGF) | 3 | 0.5% |
| Secondary graft failure (SGF) | 6 | 1.0% |
| Rejection | 7 | 1.2% |
| Re-transplantation* | 13 | 2.2% |
| Re-DMEK | 7 | 1.2% |
| Secondary DSEK | 5 | 0.8% |
| Secondary PK | 3 | 0.5% |
PI - peripheral iridectomy; TASS - toxic anterior segment syndrome; AC - anterior chamber; DM - Descemet membrane; *Two eyes required re-transplantation twice
Discussion
In this study, we evaluated the clinical outcomes of consecutive 600 eyes of standardize DMEK procedure performed by a single surgeon with a follow up ranging between 3 months to 3 years postoperatively. The patients included were more of the heterogeneous cohort with different endothelial diseases and some of them were with pre-existing comorbidities.
Our study showed that overall 49.2% of eyes achieved a BSCVA of ≥20/25 after 12 months. If we exclude the eyes with significant comorbidities (n = 69) during this period, these figures would have been 60.3%. Previous studies by different authors also showed better results revealing that up to 75% of DMEK eyes may achieve BSCVA ≥20/25.[5,6,16,18,19,20] We had a different patient population in this series with the majority of our patients being PBK/PCE and advanced FECD with presenting BSCVA of ’LP to < 20/200’ in 57.2% which was different from western reports.[5,6,7,8,19] In contrast to visual outcomes following DSEK/DSAEK and ultrathin DSAEK, which reported continuous improvement in BSCVA up to 3 years, visual acuity after DMEK was stabilized after 3 months in our series, confirming that DMEK allows the fastest visual rehabilitation among all EK procedures.[21,22,23]
Postoperative mean ECD at 3, 6, 12, and 24 months in this series was higher compared to other series. This is due to the fact that we used the highest quality of donors with mean ECD of 2884 ± 178 cells/mm2. The other probable reasons are early post-mortem use of donor tissue, in-house supply of donor corneas without any shipping, surgeon’s preparation of DMEK-graft, and no-touch technique during donor manipulation. For the same reasons, the mean ECL at 3, 6, 12, and 24 months postoperatively in this series were less compared to other studies.[5,6,7,8,19,20,21,24,25] In a small series, Bhandari et al. also showed ECL was only 24% after 6 months postoperatively and they explained that the preparation by the surgeon was important and manipulation was less traumatic.[12] Zeidenweber et al. showed that cell loss in different types of eye bank prepared DMEK tissues caused during shipping was 15% to 18%.[13] Other authors also showed similar cell loss after experimental shipping, and in their study the average damage caused by pre-stripping alone was 9.3 ± 5.9%.[14,26]
There were fewer complications in this series. We had < 10% DM detachment with rebubbling rate in 3.8% eyes which was much less than the previously published reports.[8,21,27,28] There are few similar reports of lower DM detachment with rebubbling rate (from 20% to 4.4%) by the other authors and they attributed that to the effect of the learning curve.[5,6,24,29] Iatrogenic primary graft failure (0.5%) and secondary donor failure (1.0%) were also less in our series than other studies.[5,6,8,18,28,29] This is probably due to multiple factors like, experienced DSEK surgeon who has already performed more than 1200 procedures in last 10 years, with past knowledge of avoiding complications; standardization of DMEK technique over time and late starter with knowledge gathered from the literature about DMEK complications and their prevention. Philips et al. recently published their comparable results with experienced DSAEK surgeon, transition to DMEK learning was less steep with minimum complications.[29] However, the allograft rejection rate was similar to previously reported range of 1% to 5% within the first postoperative year after DMEK.[6,8,19,30,31,32] Moreover, in contrast to other studies,[6,19,24,30] repeat transplantation procedures in our series were also less, because of less significant complications such as DM detachment, PGF, and SGF.
There are a few important highlighting points in this large series. Firstly, we used both MKM- and CSM- preserved corneas for the harvesting of donor DMEK-graft. The costs of these media, which are suitable for the developing countries are less than that of the Optisol-GS medium (Bausch and Lomb, Rochester NY, USA). Bhandari et al. used only Cornisol-preserved donor corneas for DM graft preparation, but not MKM-preserved corneas.[11,12] The published in-vitro study showed that there is no difference in endothelial viability between the donor corneas stored in Cornisol and Optisol-GS media up to 14 days of storage.[15] Secondly, in our patient populations, we have higher percentage of FECD patients (36.3%) than the other studies from India (5 to 10%).[11,33] Even then, most of our patients presented late with presenting BSCVA of < 20/200 (76.8%) in advance diseases. Thirdly, majority of these eyes were with darker iris and relatively shallow anterior chamber which were different from the eyes of western world.[34,35] In these eyes, DM-graft manipulation in the anterior chamber is relatively difficult. Because the edges of DM-scroll are not easily visible through hazier cornea against darker iris background. That is why ’S’ mark (or ’F’ mark) on DM-side during graft preparation is very important for right orientation of DM-graft after donor unfolding. The strengths of this study are a large sample size with heterogenous cohort, the use of uniform surgical technique, and good follow-up data. However, the major limitation of this study that we have not segregated data as per the indications for analysis. The other limitations are: it is a single-center, single-surgeon, retrospective noncomparative study.
Conclusion
To our knowledge, this is the largest series from India with the most heterogeneous cohort of DMEK patients with different etiologies performed by a single surgeon. Our results suggest that DMEK is a safe and effective procedure for endothelial diseases with encouraging surgical and visual outcomes. In addition, complications are less observed. ECD and ECL are acceptable with a low rejection rate to make DMEK an attractive alternative to DSEK/DSAEK. Further long-term studies are required to assess the survival of DMEK grafts in various endothelial diseases including the complicated cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Price MO, Price FW., Jr Endothelial keratoplasty-A review. Clin Experiment Ophthalmol. 2010;38:128–40. doi: 10.1111/j.1442-9071.2010.02213.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee B, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: Safety and outcomes: A report by The American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–30. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Melles GR, Ong TS, Ververs B, van der Wees J. Descemet Membrane Endothelial Keratoplasty (DMEK) Cornea. 2006;25:987–90. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 4.Baydoun L, Müller T, Lavy I, Parker J, Rodriguez-Calvo-de-Mora M, Liarakos VS, et al. Ten-year clinical outcome of the first patient undergoing descemet membrane endothelial keratoplasty. Cornea. 2017;36:379–81. doi: 10.1097/ICO.0000000000001111. [DOI] [PubMed] [Google Scholar]
- 5.Dapena I, Ham L, Droutsas K, van Dijk K, Moutsouris K, Melles GR. Learning curve in Descemet’s membrane endothelial keratoplasty: First series of 135 consecutive cases. Ophthalmology. 2011;118:2147–54. doi: 10.1016/j.ophtha.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Calvo-de-Mora M, Quilendrino R, Ham L, Liarakos VS, van Dijk K, Baydoun L, et al. Clinical outcome of 500 consecutive cases undergoing Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2015;122:464–70. doi: 10.1016/j.ophtha.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Hamzaoglu EC, Straiko MD, Mayko ZM, Sáles CS, Terry MA. The First 100 eyes of standardized descemet stripping automated endothelial keratoplasty versus standardized descemet membrane endothelial keratoplasty. Ophthalmology. 2015;122:2193–9. doi: 10.1016/j.ophtha.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Deng SX, Lee WB, Hammersmith KM, Kuo AN, Li JY, Shen JF, et al. Descemet membrane endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmology. 2018;125:295–310. doi: 10.1016/j.ophtha.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 9. [Last accessed on 2019 Aug 20]. Avaialable from: https://restoresight.org/what-we-do/publications/statistical-report/2018/
- 10.Basak SK. Descemet stripping and endothelial keratoplasty in endothelial dysfunctions: Three-month results in 75 eyes. Indian J Ophthalmol. 2008;56:291–6. doi: 10.4103/0301-4738.41412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhandari V, Reddy JK, Relekar K, Prabhu V. Descemet’s stripping automated endothelial keratoplasty versus Descemet’s membrane endothelial keratoplasty in the fellow eye for Fuchs’ endothelial dystrophy: A retrospective study? Biomed Res Int. 2015:750567. doi: 10.1155/2015/750567. doi: 10.1155/2015/750567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhandari V, Reddy JK, Chougale P. Descemet’s membrane endothelial keratoplasty in South Asian population. J Ophthalmic Vis Res. 2016;11:368–71. doi: 10.4103/2008-322X.194072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeidenweber DA, Tran KD, Sales CS, Wehrer SW, Straiko MD, Terry MA. Pre-stained and preloaded DMEK grafts: An evaluation of tissue quality and stain retention. Cornea. 2017;36:1402–7. doi: 10.1097/ICO.0000000000001329. [DOI] [PubMed] [Google Scholar]
- 14.Newman LR, DeMill DL, Zeidenweber DA, Mayko ZM, Bauer AJ, Tran KD, et al. Preloaded descemet membrane endothelial keratoplasty donor tissue: Surgical technique and early clinical results. Cornea. 2018;37:981–6. doi: 10.1097/ICO.0000000000001646. [DOI] [PubMed] [Google Scholar]
- 15.Basak S, Prajna NV. A prospective, in vitro, randomized study to compare two media for donor corneal storage. Cornea. 2016;35:1151–5. doi: 10.1097/ICO.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 16.Basak SK, Basak S, Pradhan VR. Outcomes of descemet membrane endothelial keratoplasty (DMEK) using Surgeon’s prepared donor DM-Roll in consecutive 100 Indian eyes. Open Ophthalmol J. 2018;12:134–42. doi: 10.2174/1874364101812010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–91. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 18.Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012;153:1082–90. doi: 10.1016/j.ajo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Peraza-Nieves J, Baydoun L, Dapena I, Ilyas A, Frank LE, Luceri S, et al. Two-year clinical outcome of 500 consecutive cases undergoing descemet membrane endothelial keratoplasty. Cornea. 2017;36:655–60. doi: 10.1097/ICO.0000000000001176. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk K, Ham L, Tse WH, Liarakos VS, Quilendrino R, Yeh RY, et al. Near complete visual recovery and refractive stability in modern corneal transplantation: Descemet membrane endothelial keratoplasty (DMEK) Cont Lens Anterior Eye. 2013;36:13–21. doi: 10.1016/j.clae.2012.10.066. [DOI] [PubMed] [Google Scholar]
- 21.Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet’s membrane endothelial keratoplasty: Prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 2011;118:2368–73. doi: 10.1016/j.ophtha.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Li JY, Terry MA, Goshe J, Davis-Boozer D, Shamie N. Three-year visual acuity outcomes after Descemet’s stripping automated endothelial keratoplasty. Ophthalmology. 2012;119:1126–9. doi: 10.1016/j.ophtha.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 23.Busin M, Madi S, Santorum P, Scorcia V, Beltz J. Ultrathin Descemet’s stripping automated endothelial keratoplasty with the microkeratome double pass technique: Two-year outcomes. Ophthalmology. 2013;120:1186–94. doi: 10.1016/j.ophtha.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Dirisamer M, Ham L, Dapena I, Moutsouris K, Droutsas K, van Dijk K, et al. Efficacy of descemet membrane endothelial keratoplasty: Clinical outcome of 200 consecutive cases after a learning curve of 25 cases. Arch Ophthalmol. 2011;129:1435–43. doi: 10.1001/archophthalmol.2011.195. [DOI] [PubMed] [Google Scholar]
- 25.Price MO, Giebel AW, Fairchild KM, Price FW., Jr Descemet’s membrane endothelial keratoplasty: Prospective multicentre study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–8. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Tran KD, Dye PK, Odell K, Galloway J, Stoeger CG, Straiko MD, et al. Evaluation and quality assessment of pre-stripped, preloaded descemet membrane endothelial keratoplasty grafts. Cornea. 2017;36:484–90. doi: 10.1097/ICO.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 27.Guerra FP, Anshu A, Price MO, Price FW. Endothelial keratoplasty: Fellow eyes comparison of descemet stripping automated endothelial keratoplasty and descemet membrane endothelial keratoplasty. Cornea. 2011;30:1382–6. doi: 10.1097/ICO.0b013e31821ddd25. [DOI] [PubMed] [Google Scholar]
- 28.Feng MT, Price MO, Miller JM, Price FW., Jr Air reinjection and endothelial cell density in descemet membrane endothelial keratoplasty: Five-year follow-up. J Cataract Refract Surg. 2014;40:1116–21. doi: 10.1016/j.jcrs.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Phillips PM, Phillips LJ, Muthappan V, Maloney CM, Carver CN. Experienced DSAEK Surgeon’s transition to DMEK: Outcomes comparing the last 100 DSAEK surgeries with the first 100 DMEK surgeries exclusively using previously published techniques. Cornea. 2017;36:275–9. doi: 10.1097/ICO.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 30.Showail M, Obthani MA, Sorkin N, Einan-Lifshitz A, Boutin T, Borovik A, et al. Outcomes of the first 250 eyes of descemet membrane endothelial keratoplasty: Canadian centre experience. Can J Ophthalmol. 2018;53:510–7. doi: 10.1016/j.jcjo.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Schlögl A, Tourtas T, Kruse FE, Weller JM. Long-term clinical outcome after descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2016;169:218–26. doi: 10.1016/j.ajo.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Hos D, Tuac O, Schaub F, Stanzel TP, Schrittenlocher S, Hellmich M, et al. Incidence and clinical course of immune reactions after descemet membrane endothelial keratoplasty: Retrospective analysis of 1000 consecutive eyes. Ophthalmology. 2017;124:512–8. doi: 10.1016/j.ophtha.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed A, Chaurasia S, Murthy SI, Ramappa M, Vaddavalli PK, Taneja M, et al. Endothelial keratoplasty: A review of indications at a tertiary eye care centre in South India. Asia Pac J Ophthalmol (Phila) 2014;3:207–10. doi: 10.1097/APO.0b013e3182a75304. [DOI] [PubMed] [Google Scholar]
- 34.Ronnie G, Ve RS, Velumuri L, Asokan R, Vijaya L. Importance of population-based studies in clinical practice. Indian J Ophthalmol. 2011;59(Suppl):S11–8. doi: 10.4103/0301-4738.73681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aung T, Nolan WP, Machin D, Seah SK, Baasanhu J, Khaw PT, et al. Anterior chamber depth and the risk of primary angle closure in East Asian populations. Arch Ophthalmol. 2005;123:527–32. doi: 10.1001/archopht.123.4.527. [DOI] [PubMed] [Google Scholar]