Abstract
Purpose
To investigate the clinical presentations and outcomes of retinoblastoma in relation to the advent of new multimodal treatments in Thailand. Patients and Methods. Retrospective case series. We evaluated the clinical presentation, staging, details of treatment, and treatment outcomes of retinoblastoma patients who were treated at Ramathibodi Hospital, Bangkok, Thailand, between January 1, 2007, and December 31, 2018. The log-rank test was used to explore clinical characteristics and treatment modalities that affected globe salvage and survival curves.
Results
This study included 124 eyes of 81 patients with retinoblastoma. Forty-three patients (53.1%) had bilateral retinoblastoma. The median age at diagnosis was 8 months (range, 1–48 months). Of 124 eyes, 9 eyes (7.3%) had extraocular retinoblastoma and 115 eyes (92.7%) had intraocular retinoblastoma, which were classified by the International Classification of Retinoblastoma (ICRB) as group A, 4 eyes (3.5%); group B, 19 eyes (16.5%); group C, 6 eyes (5.2%); group D, 31 eyes (27%); and group E, 56 eyes (47.8%). Treatment included systemic chemotherapy, intra-arterial chemotherapy, ruthenium-106 plaque brachytherapy, external beam radiation therapy, cryotherapy, transpupillary thermotherapy, subtenon chemotherapy, and intravitreal chemotherapy. At the median follow-up period of 38.4 months (range, 0.2–148.2 months), the overall globe salvage rate of intraocular retinoblastoma was 51.7%. For unilateral retinoblastoma, globe salvage rate was 37.5% (group B, 100%; group C, 100%; group D, 50%; and group E, 18.8%). For bilateral intraocular retinoblastoma, the globe salvage rate was 57.8% (group A, 100 %; group B, 94.4%; group C, 100%; group D, 64.7%; and group E, 28.2%). The overall survival rate was 93.8%.
Conclusions
Recent advanced treatment modalities have improved the probability of globe salvage. However, enucleation remains an important life-saving intervention in many advanced cases.
1. Introduction
Retinoblastoma is the most common primary intraocular malignant tumor in children [1], with a global incidence of approximately 7,202–8,102 annually [2]. Of these patients, approximately 40% are in Asia-Pacific countries [3]. In Thailand, the nation-wide multicenter population-based prospective study of the incidence and survival rate of childhood cancer from Thai Pediatric Oncology Group (ThaiPOG) reported 97 cases of retinoblastoma diagnosed during the year 2003–2005, placing retinoblastoma as the 7th most common childhood cancer in Thailand, with the incidence of 3.1 per million population [4]. The predicted incidence of retinoblastoma in Thailand, calculated from 67 million population, is 41 children in 2013 and 2023 [3]. The overall survival probability at 5 years of retinoblastoma in Thailand from nation-wide study was 73% [4]. However, there were different treatment outcomes among each center and region of the country. Currently, there are 7 centers that provide treatment for retinoblastoma in Thailand, 4 in Bangkok (capital city), and 3 in each part of Thailand, north, northeast, and south. Previous report from 3 cancer centers in northern, northeastern, and southern Thailand during 1990 and 2009, which included 75 retinoblastoma patients, showed the survival rate of 40%, 50%, and 75%, respectively [5]. Another report from a single institute of Bangkok during 1997 to 2006, which included 90 retinoblastoma patients, showed the survival rate of 85% [6]. Numerous studies worldwide, including Asia [7–30] and Thailand [4–6, 31–34], have described the clinical manifestations and treatment outcomes of retinoblastoma, but these studies did not use IAC, brachytherapy, or intravitreal chemotherapy as part of retinoblastoma treatment [5–8, 11–13, 17–19, 23–25, 30–34]. Therefore, our study was aimed to fill in the gap in investigating clinical presentations and treatment outcomes of retinoblastoma in relation to the advent of recent treatment modalities in Thailand.
2. Materials and Methods
2.1. Patients and Study Design
This retrospective study was approved by the institutional review board of Faculty of Medicine, Ramathibodi Hospital (ID 02-60-56). We evaluated patients diagnosed with retinoblastoma between January 1, 2007, and December 31, 2018, at Ramathibodi Hospital, Bangkok, Thailand. Those with inadequate data on clinical presentation, tumor staging, and treatment modality were excluded. Demographic data included sex, ethnicity, age of onset, lag time from first presentation to diagnosis, laterality, family history of retinoblastoma, presenting signs and symptoms, follow-up time, and details of primary and adjunctive treatments were obtained from patients' medical record and analyzed statistically.
2.2. Staging and Treatment
Each patient underwent a complete ophthalmic examination under anesthesia, which included binocular indirect ophthalmoscopy with 360-degree indentation, Schiotz tonometer, and B-scan ultrasound. Fundus imaging and fluorescein angiography were performed using a wide-angle contact fundus camera (RetCam 3, Clarity Medical Systems, Inc., Pleasanton, CA, USA). Magnetic resonance imaging of the brain and orbits was performed in all patients to identify the presence of extraocular extension and intracranial tumors (trilateral retinoblastoma). Disease staging was classified according to International Retinoblastoma Staging System [35], and eyes with intraocular retinoblastoma were classified according to the International Classification of Retinoblastoma (ICRB) [36].
Treatment was selected based on disease stage, laterality, tumor location, visual prognosis, and input from the patients' families. For unilateral intraocular retinoblastoma, patients in ICRB groups A and B with macula- and/or papilla-sparing tumors were locally treated with cryotherapy or transpupillary thermotherapy, depending on tumor location. For ICRB group B patients with macular and/or papillary involvement and patients in ICRB group C, some patients of ICRB group D were treated with chemoreduction (CRD) or IAC. Risks and benefits of CRD and IAC were discussed in details with parents. Treatment decision was based on age of patients at the time of treatment, the feasibility of catheterization during IAC, and input from patients' families. For CRD, we followed the chemotherapy protocol from Children's Hospital of Philadelphia (CHOP) retinoblastoma [37]. The CRD regimen consisted of combined intravenous chemotherapy (carboplatin-etoposide-vincristine regimen), as well as local treatments such as cryotherapy, transpupillary thermotherapy, and plaque brachytherapy. Intravenous chemotherapy was given every 3-4 weeks for a total of six cycles. The detailed chemotherapy regimens are shown in Table 1. For IAC, we used melphalan, carboplatin, and topotecan, as described in our recent study [38]. Monthly examination under anesthesia was performed during the course of the treatment to evaluate treatment response. Primary enucleation was recommended in some unilateral ICRB group D and all unilateral ICRB group E. In case of procedure refusal, IAC or IAC combined with CRD was proposed as an alternative treatment. Each enucleated eye was examined meticulously by a histopathologist trained in the evaluation of retinoblastoma globes to identify high-risk histopathological features, including postlaminar optic nerve involvement, choroidal involvement with a diameter greater than 3 mm, anterior chamber involvement, and/or the involvement of both the optic nerve and choroid. Notably, for bilateral retinoblastoma patients, the primary treatment was CRD, with IAC reserved as an adjunctive treatment for cases of incomplete tumor response with CRD.
Table 1.
| Agents/course interval | Regimens | ||
|---|---|---|---|
| Standard dose (BW < 12 kg) | Intensified dose for ICRB groups D and E (BW < 12 kg) | High dose for extraocular retinoblastoma | |
| Vincristine | 1.5 mg/m2/day on day 1 (0.05 mg/kg/day) | 1.5 mg/m2/day on day 1 (0.05 mg/kg/day) | 0.025 mg/kg/day on day 1 |
|
| |||
| Etoposide | 150 mg/m2/day on days 1-2 (5 mg/kg/day) | 180 mg/m2/day on days 1-2 (6 mg/kg/day) | 12 mg/kg/day on days 1-2 |
|
| |||
| Carboplatin | 560 mg/m2/day on day 1 (18.6 mg/kg/day) | 420 mg/m2/day on days 1-2 (14 mg/kg/day) | 28 mg/kg/day on day 1 |
|
| |||
| Course interval | Continue every 4 weeks for a total of six cycles | Continue every 4 weeks for a total of six cycles | Continue every 3 weeks for a total of six cycles |
ICRB = International Classification of Retinoblastoma.
Intravitreal chemotherapy with melphalan or methotrexate was reserved for eyes with vitreous seeds, which poorly respond to systemic chemotherapy and/or IAC [39, 40]. Safety-enhancement procedures as previously published, including ultrasonographic biomicroscopy surveillance of the injection site, lowering of intraocular pressure by paracentesis, cryotherapy at the injection site [41], and ocular surface irrigation with sterile water [42], were performed to prevent extraocular extension of the tumor.
For patients with orbital retinoblastoma, high-dose systemic chemotherapy (carboplatin-etoposide-vincristine regimen) was administered for 3–6 cycles, followed by enucleation or exenteration, external beam radiation therapy (EBRT), and adjuvant chemotherapy, for a total of 12 cycles, as previously reported [43].
2.3. Statistical Analysis
Categorical variables were compared using Pearson chi-squared or Fisher's exact tests. The Kaplan–Meier method was used to estimate globe salvage and survival. The log-rank test was used to explore clinical characteristics and treatment modalities that affected globe salvage and survival curves. Univariate and multivariate Cox regression analyses were used to calculate unadjusted and adjusted hazard ratios. All statistical analyses were performed using Stata, version 15.0. P < 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
Of 86 patients, 5 were excluded from the study due to incomplete data. Thus, 81 patients (124 eyes) were included in the analysis. Forty (49.4%) patients were male. The median age at diagnosis was 8 months (range, 1–48 months). A total of 38 (46.9%) patients had unilateral disease, while 43 (53.1%) had bilateral disease. The median age of onset for bilateral disease was significantly lower than for unilateral disease (5 months vs 17 months, P < 0.001). The median lag time between symptom onset and visit to the oncology service was 6 weeks (range, 1–80 weeks). In our study, there were 4 patients (4.9%) with familial retinoblastoma from 3 families. Two patients were siblings from the same family, and 2 patients from 2 families had parents with retinoblastoma.
Leukocoria was the most common presenting symptom (n = 62 patients, 76.5%), followed by strabismus (n = 8 patients, 9.9%), eye screening (n = 4 patients, 4.9%), buphthalmos (n = 3 patients, 3.7%), orbital cellulitis (n = 2 patients, 2.5%), red eye (n = 1 patient, 1.2%), and poor visual behavior (n = 1 patient, 1.2%). Overall, 84% were treatment-naïve, while 16% were treated initially from other centers. The overall median follow-up time was 38.4 months (range, 0.2–148.2 months), which were 4.7 months (range, 0.2–9.9 months) for dead patients and 46.4 months (range, 8.4–148.2 months) for survived patients.
3.2. Stage
Of the 81 patients, 88.9% (n = 72 patients, 115 eyes) had intraocular retinoblastoma, which was unilateral in 32 (44.4%) patients and bilateral in 40 (55.6%) patients. Of 115 eyes, 4 (3.5%), 19 (16.5%), 6 (5.2%), 31 (27%), and 55 (47.8%) were classified as ICRB groups A, B, C, D, and E, respectively. For the unilateral tumor group (32 eyes, 27.8%), 1 (3.13%), 1 (3.13%), 14 (43.8%), and 16 (50%) were classified as ICRB groups B, C, D, and E, respectively. For the bilateral tumor group (83 eyes, 72.2%), 4 (4.8%), 18 (21.7%), 5 (6%), 17 (20.5%), and 39 (47%) were classified as the ICRB groups A, B, C, D, and E, respectively. Notably, the majority of eyes with intraocular retinoblastoma in our study exhibited advanced stage of disease, which was classified as ICRB groups D and E in 86 eyes (74.8%).
Extraocular extension, diagnosed on the basis of clinical and radiological manifestations, was found in 9 of 81 patients (11.1%). Of these nine patients, four (44.4%) had overt orbital disease, while 5 (55.6%) had radiologically detected extraocular extension. Lumbar puncture and bone marrow biopsy were performed in all patients with extraocular tumors. No patients had central nervous system or distant metastasis at initial presentation.
There were no statistically significant differences in median age of onset, median lag time, sex, and laterality between intraocular and extraocular tumor groups.
3.3. Treatment
Detailed treatment modalities of the studied eyes are shown in Tables 2 and 3. For the unilateral intraocular tumor group (32 eyes, 27.8%), 23 eyes (71.9%) were treated with globe salvage therapy. Primary enucleation was performed in 9 eyes (28.1%). Secondary enucleation was performed in 11 eyes (34.4%). For the bilateral intraocular tumor group (83 eyes, 72.2%), 69 eyes (83.1%) were treated with globe salvage therapy. Primary enucleation was performed in 14 eyes (16.9%). Secondary enucleation was performed in 21 eyes (25.3%). High-risk pathological features were found in 12 enucleated patients (21.8%), which all received postenucleation adjuvant chemotherapy. There was no local recurrence or systemic metastasis in this group of patients at a median follow-up time of 27.4 months (range, 0.4–107.7 months).
Table 2.
Treatment modalities and globe salvage rates for intraocular tumor according to International Classification of Retinoblastoma (ICRB).
| Treatment modalities, n = 115 | ICRB group, eyes (globe salvage, %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | ||||||
| Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | |
| CRD | — | 4 (100%) | 1 (100%) | 9 (100%) | — | 2 (100%) | — | 9 (66.7%) | 2 (0%) | 19 (47.4%) |
| CRD + plaque | — | — | — | 2 (100%) | 1 (100%) | — | — | — | — | |
| CRD + EBRT | — | — | — | 1 (100%) | — | — | — | 1 (0%) | — | |
| CRD + IAC | — | — | — | 2 (100%) | — | 1 (100%) | 4 (75%) | 4 (100%) | 3 (0%) | 6 (33.3%) |
| CRD + IAC + IVT | — | — | — | 1 (100%) | — | 2 (100%) | 1 (100%) | — | — | |
| CRD + IAC + plaque | — | — | — | — | — | — | 4 (75%) | 1 (0%) | — | |
| CRD + IVT + plaque | — | — | — | 2 (50%) | — | — | — | — | — | |
| CRD + IAC + IVT + plaque | — | — | — | 1 (100%) | — | — | 1 (0%) | — | — | |
| IAC | — | — | — | — | — | — | 1 (0%) | 1 (100%) | 3 (67.7%) | 1 (0%) |
| IAC + IVT | — | — | — | — | — | — | — | — | 1 (100%) | |
| IAC + IVT + plaque | — | — | — | — | — | — | 1 (0%) | — | ||
| Primary enucleation (in our center/from referral hospital) | — | — | — | — | — | — | 2 (1/1) | 1 (0/1) | 7 (7/0) | 13 (8/5) |
| Secondary enucleation (in our center/from referral hospital) | — | — | — | 1 | — | — | 5 (5/0) | 5 (5/0) | 6 (6/0) | 15 (14/1) |
|
| ||||||||||
| Total | 0 | 4 (100%) | 1 (100%) | 18 (94.4%) | 1 (100%) | 5 (100%) | 14 (50%) | 17 (64.7%) | 16 (18.8%) | 39 (28.2%) |
ICRB = International Classification of Retinoblastoma; CRD = chemoreduction; IAC = intra-arterial chemotherapy; EBRT = external beam radiation therapy; IVT = intravitreal chemotherapy.
Table 3.
Treatment modalities for intraocular and extraocular tumors according to the International Retinoblastoma Staging System (IRSS).
| Treatment modalities, n = 124 | IRSS staging, eyes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | ||||||
| Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | |
| Globe salvage treatment1 | 12 | 48 | — | — | — | — | — | — | — | — |
| CRD + enucleation | — | — | 3 | 15 | — | — | — | — | — | — |
| CRD + IAC + enucleation | — | — | 4 | 4 | — | — | — | — | — | — |
| CRD + EBRT + enucleation | — | — | — | 1 | — | — | 1 | — | 1 | — |
| CRD + IAC + plaque + enucleation | — | — | 1 | 1 | — | — | — | — | — | — |
| CRD + IAC + IVT + plaque + enucleation | — | — | 1 | — | — | — | — | — | — | — |
| CRD + IVT + plaque + enucleation | — | — | — | 1 | — | — | — | — | — | — |
| CRD + exenteration | — | — | 2 | — | — | — | — | — | — | — |
| CRD + exenteration + EBRT | — | — | — | — | — | — | 1 | — | — | — |
| IAC + enucleation | — | — | 2 | 1 | — | — | — | — | — | — |
| IAC + IVT + plaque + enucleation | — | — | 1 | — | — | — | — | — | — | — |
| Primary enucleation (in our center/from referral hospital) | — | — | 9 (8/1) | 14 (8/6) | — | — | — | — | — | 1 (0/1) |
|
| ||||||||||
| Total | 12 | 48 | 23 | 37 | 0 | 0 | 2 | 0 | 1 | 1 |
1Detailed treatment modalities are listed in Table 1. IRSS = International Retinoblastoma Staging System; CRD = chemoreduction; IAC = intra-arterial chemotherapy; EBRT = external beam radiation therapy; IVT = intravitreal chemotherapy.
3.4. Treatment Outcome and Survival Outcomes
With the availability of new treatment modalities at our center, the overall globe salvage rate for the intraocular retinoblastoma group was 51.7% (groups A and C, 100%; group B, 94.7%; group D, 58%; and group E, 25%). Regarding to laterality, the globe salvage rate was 37.5% in the unilateral intraocular retinoblastoma group (group B, 100%; group C, 100%; group D, 50%; and group E, 18.8%) and 57.8% for the bilateral intraocular retinoblastoma group (group A, 100%; group B, 94.4%; group C, 100%; group D, 64.7%; and group E, 28.2%). Five patients died in our study. The overall survival rate was 93.8%. The detailed characteristics and treatment course of deceased patients are summarized in Supplement Table 1.
Kaplan–Meier analysis included 104 of 115 eyes with intraocular tumors, 29 eyes of unilateral intraocular tumor, and 75 eyes of bilateral intraocular tumor (Figure 1(a)); the remaining eyes were excluded because enucleation was initially performed at other center. The probabilities of globe salvage for unilateral and bilateral groups were 54% and 68% at 1 year, 41% and 64% at 2 years, and 34% and 62% at 5 years, respectively. Figures 1(b) and 1(c) show the probability of globe salvage based on ICRB classification. Kaplan–Meier analysis showed a 5-year survival rate of 93.5%.
Figure 1.
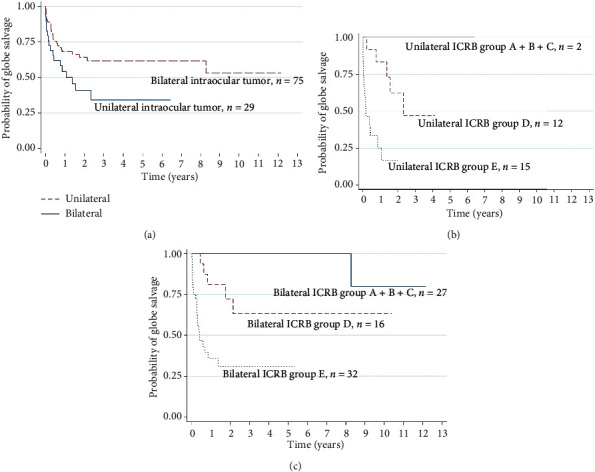
Kaplan–Meier survival analysis of globe salvage in intraocular tumors: (a) including all eyes (unilateral vs. bilateral); (b, c) unilateral and bilateral: based on the International Classification of Retinoblastoma (ICRB).
The hazard ratios of globe salvage according to patient characteristics and treatment modalities are shown in Figure 2. For all eye analysis (Figure 2(a)), Cox regression analysis showed that the bilateral group had better globe salvage outcome (HR: 0.46, P=0.007). However, there were no statistically significant differences in globe salvage outcome with respect to other characteristics and treatment modalities by all analysis (all eyes, unilateral tumor and bilateral tumor) (Figures 2(a) and 2(c)).
Figure 2.
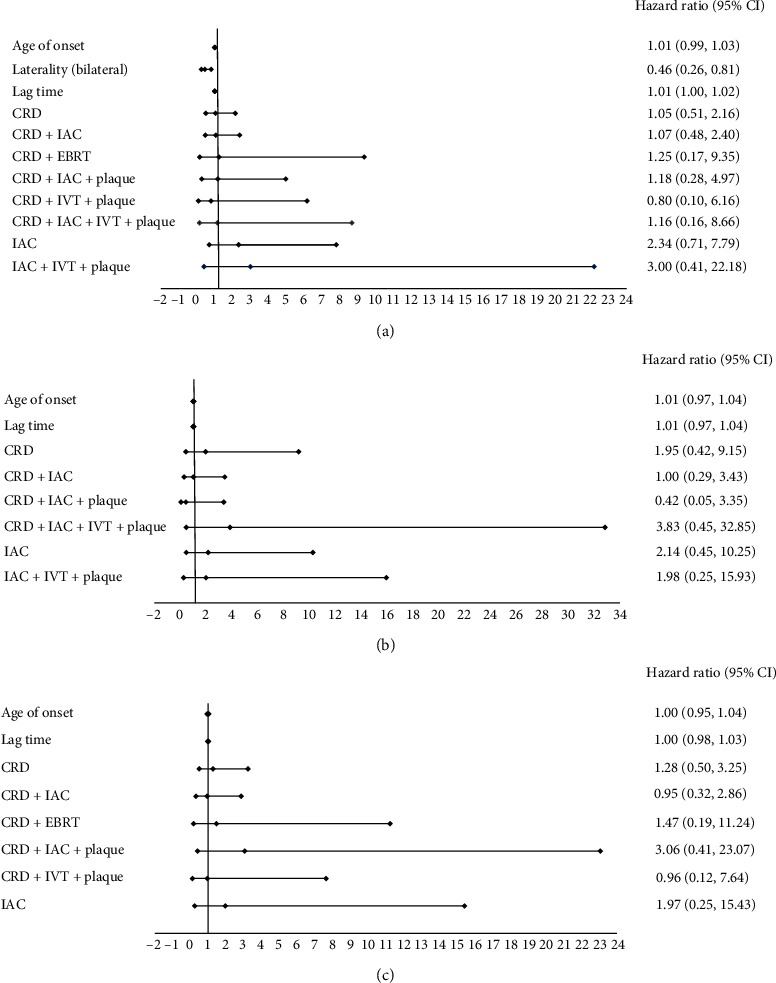
Hazard ratio of globe salvage according to patient characteristics and treatment modalities: (a) including all eyes (unilateral vs. bilateral); (b, c) unilateral and bilateral: based on the International Classification of Retinoblastoma (ICRB). CRD = chemoreduction; IAC = intra-arterial chemotherapy; EBRT = external beam radiation therapy; IVT = intravitreal chemotherapy.
4. Discussion
Several studies have shown that the clinical characteristics and treatment outcomes of retinoblastoma vary worldwide (Table 4). In this study, we investigated the clinical presentations and outcomes of retinoblastoma in relation to the advent of new multimodal treatments in Thailand. The median age of onset was 8 months (range, 1–48 months), which was comparable to a study from Japan (13 months) [19]. However, an older age of onset has been reported in studies from other nations, ranging from 13 to 36 months [6–8, 10, 12, 13, 17, 19, 23–25, 27, 29, 30, 45–49, 51]. Although our study showed an approximately equal proportion of unilateral and bilateral disease (46.9% and 53.1% for unilateral and bilateral disease, respectively), studies from the United States [52] and India [8] have revealed higher rates of unilateral disease than bilateral disease. Similar to other studies [6–8, 12, 13, 17, 22–25, 53, 54], the median age at diagnosis in our patients with bilateral disease was significantly lower than that of patients with unilateral disease (5 months vs 17 months, P < 0.001). The median lag time from first presentation until detection by an ocular oncologist was 5 weeks (range, 1–80 weeks), which was shorter than the median of 3 months reported in a study from India [13]. The shorter lag time may reflect increasing public awareness and improvements in our referral systems. No sex bias was noted in our patients, which was consistent with many previous studies [8, 54]. However, a male predominance has been reported in some studies [13], which might be attributed to the lack of attention to female children in those countries, particularly in rural areas [13]. Similar to several prior studies [5, 7–10, 12, 13, 17, 19, 23–25, 27, 29, 55], the most common presenting symptom in our study was leukocoria (76.5%), followed by strabismus (9.9%). As an initial sign, proptosis was found in 40% of cases in a study from Nepal [30], whereas none of our patients exhibited proptosis at their initial visit. Most retinoblastoma patients in developed countries exhibit intraocular tumors at presentation, while patients with extraocular tumors are rare (3%-4%) [19, 56]. In contrast, extraocular extension is commonly found in developing countries in Asia, Africa, and South America (approximately 27%–69%) [10, 13, 30, 45, 48, 49]. In our study, extraocular retinoblastoma was found in 9.8% of all cases. Our analysis showed that the median lag time was not correlated with the presence of extraocular extension. This might be related to the limited number of cases with extraocular extension in our study. Among patients with intraocular retinoblastoma, the number of patients presenting at advanced stages (ICRB groups D or E) are higher in developing countries including Thailand [6–8, 10, 12–14, 27, 29, 48]. Therefore, a major challenge in our country is the implementation of an early detection program to minimize the progression of retinoblastoma to advanced stages and allow for the treatment at earlier stages of disease and have better treatment outcomes. The main goals of retinoblastoma treatment, in descending order of importance, are to save the life, globe, and vision of the patient. In order to achieve the treatment goals, we treated our patients based on laterality, tumor staging, tumor location, visual potential, and input from patients' families.
Table 4.
Summary of retinoblastoma patient characteristics and treatment outcomes in the published literature1.
| Author | Year of publication | Country | No. of patients/eyes | Median/mean age of onset (months) | Most common presenting symptom (%) | % eyes with advanced ICRB groups D and E | % patients with EOE | Patient survival rate | Globe salvage rate for intraocular tumor | Follow-up time (median or mean) |
|---|---|---|---|---|---|---|---|---|---|---|
| Our study | 2018 | Thailand | 81/124 | 8 (1–48) | Leukocoria (76.5%) | 70.2% | 6.5% | 93.8% | 51.7% | 38 (0.2–148) months |
| Francis et al. [44] | 2018 | USA | 46/92 | 5.48 (median) (0.33–27.9) | NA | 48.9% | 6.52% | 97.83% | 91.30% | 38.7 (6.9–60.9) months |
| Goolam et al. [45] | 2018 | South Africa | 245/330 | 32.6 (mean) ± 26.8 | Leukocoria (37%) | 19% IRSS stage 0-1 | 69.39% | 58% | NA | 71.9 (3–238) months |
| Kaliki et al. [8] | 2017 | India | 1457/2074 | 24 (<1–370) | Leukocoria (75%) | 73% | 9% (eye) | 92% | 45% for all eyes | 30 (3–234) months |
| Al Hasan et al. [9] | 2017 | Syria | 37/65 | Age 4 months to 1 year in 40.6% | Leukocoria (57%) | NA | 13.50% | NA | NA | NA |
| Soliman et al. [7] | 2017 | Egypt | 47/70 | 24 (2–49) | Leukocoria (96%) | 64% | 0% | 100% | NA | NA |
| Berry et al. [46] | 2017 | USA | 294/345 | 19.8 (mean) | NA | Group D 47.8%; group E 52.2% | 0.9% (patient) | 98.5 | 40.3% | 86.3 (mean) ± 62.5 months |
| Li et al. [11] | 2016 | Taiwan | 154/NA | NA | NA | NA | NA | 83% | NA | NA |
| Gao et al. [12] | 2016 | China | 253/303 | 21 (0–611) | Leukocoria (71%) | 90% | 9% | 91% | 21.70% | 16 (0.3–119) months |
| Selistre et al. [10] | 2016 | Brazil | 140/187 | 23.5 (mean) | Leukocoria (74%) | 78% of all eyes | 36.40% | 86% | 29.30% | 323 (300–346) months |
| Chawla et al. [13] | 2016 | India | 600/794 | 21 (1–150) | Leukocoria (83%) | 78% | 27.70% | 76% | 28.2 | 21 (1–60) months |
| Lumbroso-Le Rouic et al. [15] | 2015 | France | 730/1049 | NA | NA | NA | NA | 98.5 | NA | 93 (median follow-up time) months |
| Gichico et al. [16] | 2015 | Kenya | 160 | NA | NA | NA | NA | 27% | NA | 37.5 (1–144) months |
| Waddell et al. [14] | 2015 | Uganda | 270/NA (181 before introduction of chemotherapy, 89 after introduction of chemotherapy) | NA | NA | 98% after introduction of chemotherapy had group E or EOE | NA | 45% (before introduction of chemotherapy) and 65% (after introduction of chemotherapy) | NA | 1–86 months |
| Okimoto et al. [19] | 2014 | Japan | 34/43 | 13 (0.7–74) | Leukocoria (97%) | 86% | 2.90% | 97% | 25.60% | 106.6 ± 53 months |
| Park et al. [18] | 2014 | Korea | 600/NA | NA | NA | NA | NA | 92% | NA | 10 years |
| da Rocha-Bastos et al. [22] | 2014 | Portugal | 46/59 | Mean age: 22.19 for unilateral, 6.92 for bilateral | Leukocoria (37%) | 54% Reese–Ellsworth IV-V | NA | 98% | 23.70% | 12 (1–33) years |
| Moreno et al. [20] | 2014 | Argentina | 438/577 | Unilateral: 35 for lower EHDI and 24 for higher EHDI. Bilateral: 11.5 for lower EHDI and 9 for higher EHDI | NA | NA | NA | 89% | NA | NA |
| Subramaniam et al. [17] | 2014 | Malaysia | 119/162 | 22 (1–123) | Leukocoria (92%) | NA | NA | 55% | NA | 1 year |
| Al-Nawaiseh I et al. [47] | 2014 | Jordan | 71/114 | 21.7 (mean) (1–276) | Leukocoria (54%) | 48% Reese–Ellsworth I–IV; 52% Reese–Ellsworth V | NA | NA | 63.2% | 26.9 (0.25–160) months |
| Lim et al. [23] | 2013 | Singapore | 51/67 | 25.7 (SD 19.9) | Leukocoria (71%) | 88% | 0% | 91% | 23.90% | 5 years |
| Nabie et al. [24] | 2012 | Iran | 40/57 | 20 (2 weeks–10.2 years) | Leukocoria (98%) | NA | NA | NA | 26.3% for all eyes | NA |
| Luna-Fineman S et al. [48] | 2012 | Central America | 171/213 | 28 (median) (1–108) | NA | 80% Reese–Ellsworth IV–V | 62% | 52% | NA | 18 (median) (1–112) months |
| Ali AAE et al. [49] | 2011 | Sudan | 25/31 | 36 (median) (8–60) | Enlarged eye (56%) | NA | 64% | 56% | NA | 15.4 (median) (9–36) months |
| Essuman et al. [27] | 2010 | Ghana | 23/27 | 24.0 ± 11.3 | Leukocoria (87%) | 87% Reese–Ellsworth V | 17% | 26% | NA | 5.7 months |
| Zhao et al. [25] | 2010 | China | 470/595 | 20 (2 weeks–10.2 years) | Leukocoria (73%) | NA | NA | NA | NA | NA |
| Atchaneeyasakul et al. [6] | 2009 | Thailand | 90/116 | 18 (0.5–80) | NA | 76% | 14% | 85% | 16% for all eyes | 27 months |
| Broaddus et al. [50] | 2009 | USA | 992/NA | NA | NA | NA | NA | 95% | NA | 5 years |
| MacCarthy et al. [28] | 2008 | Great Britain | 1576/2156 | NA | NA | NA | NA | Unilateral cases diagnosed: 1963–1967, 85%; 1998–2002, 97%. Bilateral cases diagnosed: 1963–1967, 88%; 1998–2002, 100% | NA | 5 years |
| Ozdemir et al. [29] | 2007 | Turkey | 91/121 | 18 (2–100) | Leukocoria (65%) | 79% Reese–Ellsworth IV-V | 20.9% | 92% | 46.3% for all eyes | 35 (4–63) months |
| Shields et al. [36] | 2006 | USA | 163/249 | NA | NA | 44% | 0 | 100% | 73.10% | 6.2 (1–10.6) years |
| Badhu et al. [30] | 2005 | Nepal | 43/47 | 3.04 ± 1.80 years | Proptosis (40%) | NA | 46.5% | 53.5% | NA | Minimum 2 years |
1Search strategy: scientific literatures on retinoblastoma treatments written in English published between 2005 and 2020 were searched using PubMed. The search terms included retinoblastoma, retinoma, retinocytoma, chemoreduction, intra-arterial chemotherapy, brachytherapy, external beam radiation therapy, intravitreal chemotherapy, enucleation, exenteration, globe salvage rate, and survival rate. The literatures from each countries/regions were selected based on well-documented treatment modalities, tumor staging, and treatment outcomes. ICRB = International Classification of Retinoblastoma; EOE = extraocular extension; NA = nonapplicable; EHDI = Extended Human Development Index.
The role of systemic chemotherapy in preventing pinealoblastoma and second neoplasm in germline retinoblastoma is controversial [57–59]. We used VEC regimen in our study for bilateral retinoblastoma patients and found that no patients in our study developed pinealoblastoma or second neoplasm during our study period. However, 5 patients died in our study, and 3 were chemotherapy-related (febrile neutropenia, chemotherapy toxicity, and chemotherapy-induced sAML). Therefore, the risks and benefits of systemic chemotherapy should be reviewed with family members before the initiation of treatment. Further studies regarding systemic chemotherapy-related complications and its role in preventing pinealoblastoma or second neoplasms are needed to identify the true risks and benefits of systemic chemotherapy in the treatment of bilateral retinoblastoma.
Because of the greater proportion of advanced retinoblastoma cases in our series, the overall globe salvage rate was 51.7%, which was much lower than that observed in developed nations [36, 44, 56]. However, globe salvage was achieved in 100% of patients in ICRB groups A and C, in 94.7% of patients in group B, in 58% of patients in group D, and in 25% of patients in group E. This was comparable to that in developed nations [36]. There was no significant association between globe salvage outcome and treatment modalities, as shown in Figure 2. This may be the result of the small sample sizes within the treatment groups.
Primary enucleation remains a life-saving intervention in advanced unilateral retinoblastoma (ICRB groups D and E) because it can effectively cure the disease, save the patients' life, reduce the risk of metastasis, reduce the number of subsequent follow-up visits, and avoid possible side effects of systemic chemotherapy or IAC. The pathological examination of enucleated eye by histopathologist trained in the evaluation of retinoblastoma globe is crucial to identify high-risk pathological features. Graduated intensity of postenucleation adjuvant chemotherapy should be given based on pathological study of each enucleated eye [60]. In Thailand and some other countries, especially in Asia, primary enucleation may be unacceptable to many patients' families. Strong advice of primary enucleation to these families can lead to treatment refusal, abandonment of the treatment, and subsequently loss of follow-up. In this situation globe salvage treatment may keep patients' families on the treatment track and make secondary enucleation more acceptable. A study from Malaysia [51] reported that most families refuse the treatment, as well as any further management, upon counseling in favor of enucleation. At our center, globe salvage treatment was offered as an alternative treatment for patients with unilateral ICRB group D or E whose families strongly refused enucleation. This was performed with the aim of preventing families from abandoning treatment altogether. All of them were informed of and accepted the risk of extraocular extension and distant metastasis. Ultimately, there was no abandonment of treatment in this group. In our study, 30 eyes had unilateral intraocular retinoblastoma group D or E. Nine eyes (30%) underwent primary enucleation, while twenty-one eyes (70%) underwent primary systemic chemoreduction and/or IAC with various adjuvant treatment modalities (Table 2). The globe salvage rate was 10 eyes (33.3%) with the median follow-up time of 26 months, and neither local recurrence nor distant metastasis was observed. However, 11 eyes (36.7%) did not respond to treatments and eventually underwent secondary enucleation as permitted by their families.
As shown in Table 4, the survival rate of retinoblastoma patients in internationally published data varies from 26% to 100%, with developed nations tending to exhibit more favorable survival outcomes than developing nations. The primary influencing factor of disease-related mortality was extraocular extension, which was generally attributed to late presentation and delay in the referral system. In our series, the overall survival rate of 93.8% was consistent with the 5-year survival estimated using Kaplan–Meier analysis. Of the five deaths recorded in our series, two were related to extraocular disease, and the remaining three were due to chemotherapy-related complications (febrile neutropenia, chemotherapy toxicity, and sAML).
The major limitation of this study was its limited scope and sample size; all data were obtained from a single referral center in Bangkok, which may not entirely represent retinoblastoma patients in Thailand. A multicenter national study and a national system of retinoblastoma registration are required to establish a nation-wide picture of this disease. Education of the public and primary health care providers, along with the implementation of a retinoblastoma screening program, should be promoted to enable earlier detection of retinoblastoma. This would enable patients to achieve better treatment outcomes. In addition, proper retinoblastoma management requires multidisciplinary cooperation, which includes medical personals, nurses, ocularists, ocular oncologists, pediatric oncologists, interventional neuroradiologists, neurosurgeons, anesthesiologists, and ocular pathologists. Because these specialized providers are not available in many areas of the country, comprehensive referral centers may provide a solution for the enhanced provision of retinoblastoma care in Thailand.
5. Conclusions
In summary, we have shown the clinical presentation and treatment outcomes of retinoblastoma patients who underwent recent multimodal treatments at our center, spanning a period of 12 years. Although the treatment of retinoblastoma remains a complex challenge in Thailand, the recent availability of advanced treatment modalities at our center has enhanced our capacity to save lives and has increased the likelihood of globe salvage. The current study fills a gap in the existing literature by reporting clinical presentations and outcomes of retinoblastoma with the recent multimodal treatments in Thailand.
Acknowledgments
This work was supported by the Ramathibodi Foundation and Children Cancer Fund under the Patronage of HRH Princess Soamsawali.
Data Availability
All the data used to support the findings of this study are included within the article and are available from the corresponding author by a reasonable request.
Disclosure
The funders had no role in design of the study, in the data collection, analysis, or interpretation of data, in the decision to publish, or in the writing of the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest pertaining to the publication of this article.
Supplementary Materials
Supplement Table 1. The detailed characteristics and treatment course of dead patients.
References
- 1.Abramson D. H. Retinoblastoma in the 20th century: past success and future challenges the Weisenfeld lecture. Investigative Ophthalmology & Visual Science. 2005;46:2683–2691. doi: 10.1167/iovs.04-1462. [DOI] [PubMed] [Google Scholar]
- 2.Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. British Journal of Ophthalmology. 2009;93(9):1129–1131. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 3.Usmanov R. H., Kivelä T. Predicted trends in the incidence of retinoblastoma in the Asia-Pacific region. Asia-Pacific Journal of Ophthalmology. 2014;3(3):151–157. doi: 10.1097/apo.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 4.Wiangnon S., Veerakul G., Nuchprayoon I., et al. Childhood cancer incidence and survival 2003-2005, Thailand: study from the Thai Pediatric Oncology Group. Asian Pacific Journal of Cancer Prevention: APJCP. 2011;12(9):2215–2220. [PubMed] [Google Scholar]
- 5.Wongmas P., Jetsrisuparb A., Komvilaisak P., et al. Incidences, trends and long term outcomes of retinoblastoma in three cancer registries, Thailand. Asian Pacific Journal of Cancer Prevention. 2015;16(16):6899–6902. doi: 10.7314/apjcp.2015.16.16.6899. [DOI] [PubMed] [Google Scholar]
- 6.Atchaneeyasakul L.-o., Wongsiwaroj C., Uiprasertkul M., Sanpakit K., Thephamongkhol K., Trinavarat A. Prognostic factors and treatment outcomes of retinoblastoma in pediatric patients: a single-institution study. Japanese Journal of Ophthalmology. 2009;53(1):35–39. doi: 10.1007/s10384-008-0614-y. [DOI] [PubMed] [Google Scholar]
- 7.Soliman S. E., Eldomiaty W., Goweida M. B., Dowidar A. Clinical presentation of retinoblastoma in Alexandria: a step toward earlier diagnosis. Saudi Journal of Ophthalmology. 2017;31(2):80–85. doi: 10.1016/j.sjopt.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaliki S., Patel A., Iram S., Ramappa G., Mohamed A., Palkonda V. A. R. Retinoblastoma in India. Retina. 2019;39(2):379–391. doi: 10.1097/iae.0000000000001962. [DOI] [PubMed] [Google Scholar]
- 9.Al Hasan A., Murad R., Zaid K., et al. Epidemiological characteristics of retinoblastoma in children attending almouassat university hospital, damascus, Syria, 2012-2016. Asian Pacific Journal of Cancer Prevention. 2017;18:421–424. doi: 10.22034/APJCP.2017.18.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selistre S. G. A., Maestri M. K., Santos-Silva P., et al. Retinoblastoma in a pediatric oncology reference center in Southern Brazil. BMC Pediatr. 2016;16:p. 48. doi: 10.1186/s12887-016-0579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S.-Y., Chen S. C.-C., Tsai C.-F., Sheu S.-M., Yeh J.-J., Tsai C.-B. Incidence and survival of retinoblastoma in Taiwan: a nationwide population-based study 1998-2011. British Journal of Ophthalmology. 2016;100(6):839–842. doi: 10.1136/bjophthalmol-2015-307211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao J., Zeng J., Guo B., et al. Clinical presentation and treatment outcome of retinoblastoma in children of South Western China. Medicine (Baltimore) 2016;95 doi: 10.1097/md.0000000000005204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawla B., Hasan F., Azad R., et al. Clinical presentation and survival of retinoblastoma in Indian children. British Journal of Ophthalmology. 2016;100(2):172–178. doi: 10.1136/bjophthalmol-2015-306672. [DOI] [PubMed] [Google Scholar]
- 14.Waddell K. M., Kagame K., Ndamira A., et al. Improving survival of retinoblastoma in Uganda. British Journal of Ophthalmology. 2015;99(7):937–942. doi: 10.1136/bjophthalmol-2014-306206. [DOI] [PubMed] [Google Scholar]
- 15.Lumbroso-Le Rouic L., Savignoni A., Levy-Gabriel C., et al. Treatment of retinoblastoma: the Institut Curie experience on a series of 730 patients (1995 to 2009) Journal Français d’Ophtalmologie. 2015;38(6):535–541. doi: 10.1016/j.jfo.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Gichigo E. N., Kariuki–Wanyoike M. M., Kimani K., Nentwich M. M. Retinoblastom in kenia. Der Ophthalmologe. 2015;112(3):255–260. doi: 10.1007/s00347-014-3123-z. [DOI] [PubMed] [Google Scholar]
- 17.Subramaniam S., Rahmat J., Rahman N. A., et al. Presentation of retinoblastoma patients in Malaysia. Asian Pacific Journal of Cancer Prevention. 2014;15(18):7863–7867. doi: 10.7314/apjcp.2014.15.18.7863. [DOI] [PubMed] [Google Scholar]
- 18.Park S. J., Woo S. J., Park K. H. Incidence of retinoblastoma and survival rate of retinoblastoma patients in Korea using the Korean National Cancer Registry database (1993-2010) Investigative Opthalmology & Visual Science. 2014;55(5):2816–2821. doi: 10.1167/iovs.14-14078. [DOI] [PubMed] [Google Scholar]
- 19.Okimoto S., Nomura K. Clinical manifestations and treatment of retinoblastoma in Kobe children’s hospital for 16 years. Journal of Pediatric Ophthalmology & Strabismus. 2014;51(4):222–229. doi: 10.3928/01913913-20140513-01. [DOI] [PubMed] [Google Scholar]
- 20.Moreno F., Sinaki B., Fandiño A., Dussel V., Orellana L., Chantada G. A population-based study of retinoblastoma incidence and survival in Argentine children. Pediatric Blood & Cancer. 2014;61(9):1610–1615. doi: 10.1002/pbc.25048. [DOI] [PubMed] [Google Scholar]
- 21.Kruger M., Reynders D., Omar F., Schoeman J., Wedi O., Harvey J. Retinoblastoma outcome at a single institution in South Africa. South African Medical Journal. 2014;104(12):859–863. doi: 10.7196/samj.8255. [DOI] [PubMed] [Google Scholar]
- 22.Bastos R. A. d. R., Silva R., Gil-da-Costa M., et al. Retinoblastoma: experience of a referral center in the North Region of Portugal. Clinical Ophthalmology. 2014;8:993–997. doi: 10.2147/opth.s59601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim F. P. M., Soh S. Y., Iyer J. V., Tan A. M., Swati H., Quah B. L. Clinical profile, management, and outcome of retinoblastoma in Singapore. Journal of Pediatric Ophthalmology & Strabismus. 2013;50(2):106–112. doi: 10.3928/01913913-20121211-01. [DOI] [PubMed] [Google Scholar]
- 24.Nabie R., Taheri N., Fard A. M., Fouladi R. F. Characteristics and clinical presentations of pediatric retinoblastoma in North-western Iran. International Journal of Ophthalmology. 2012;5(4):510–512. doi: 10.3980/j.issn.2222-3959.2012.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J., Li S., Shi J., Wang N. Clinical presentation and group classification of newly diagnosed intraocular retinoblastoma in China. British Journal of Ophthalmology. 2011;95(10):1372–1375. doi: 10.1136/bjo.2010.191130. [DOI] [PubMed] [Google Scholar]
- 26.Abdu L., Malami S. Clinicopathological pattern and management of retinoblastoma in Kano, Nigeria. Annals of African Medicine. 2011;10(3):214–219. doi: 10.4103/1596-3519.84705. [DOI] [PubMed] [Google Scholar]
- 27.Essuman V., Ntim-Amponsah C. T., Akafo S., et al. Presentation of retinoblastoma at a paediatric eye clinic in Ghana. Ghana Medical Journal. 2010;44:101–105. doi: 10.4314/gmj.v44i1.68850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacCarthy A., Birch J. M., Draper G. J., et al. Retinoblastoma: treatment and survival in great britain 1963 to 2002. British Journal of Ophthalmology. 2009;93(1):38–39. doi: 10.1136/bjo.2008.139626. [DOI] [PubMed] [Google Scholar]
- 29.Ozdemir H., Tacyildiz N., Unal E., Yavuz G., Ugur H., Gunduz K. Clinical and epidemiological characteristics of retinoblastoma: correlation with prognosis in a Turkish pediatric oncology center. Pediatric Hematology and Oncology. 2007;24(3):221–231. doi: 10.1080/08880010601107623. [DOI] [PubMed] [Google Scholar]
- 30.Badhu B., Sah S. P., Thakur S. K., et al. Clinical presentation of retinoblastoma in Eastern Nepal. Clinical and Experimental Ophthalmology. 2005;33(4):386–389. doi: 10.1111/j.1442-9071.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- 31.Buaboonnam J., Narkbunnam N., Vathana N., et al. Outcomes of pediatric retinoblastoma treated with ICEV regimen: a single-center study. Pediatric Hematology and Oncology. 2019;36(2):73–81. doi: 10.1080/08880018.2019.1600083. [DOI] [PubMed] [Google Scholar]
- 32.Wongmeerit P., Suwanrungruang K., Jetsrisuparb A., Komvilaisak P., Wiangnon S. Trends in survival of childhood cancers in a university hospital, northeast Thailand, 19932012. Asian Pacific Journal of Cancer Prevention: APJCP. 2016;17(7):3515–3519. [PubMed] [Google Scholar]
- 33.Hathirat P., Kunavisarut S., Chuansumrit A., Pochanukul L., Simaroj P., Isarangkura P. Chemotherapy in patients with retinoblastoma. Journal of the Medical Association of Thailand. 1993;76(Suppl 2):85–91. [PubMed] [Google Scholar]
- 34.Pochanugool L., Kunavisarut S., Hathirat P., Dangprasert S. The role of radiation therapy in retinoblastoma. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet. 1994;77(8):393–399. [PubMed] [Google Scholar]
- 35.Chantada G., Doz F., Antoneli C. B. G., et al. A proposal for an international retinoblastoma staging system. Pediatric Blood & Cancer. 2006;47(6):801–805. doi: 10.1002/pbc.20606. [DOI] [PubMed] [Google Scholar]
- 36.Shields C. L., Mashayekhi A., Au A. K., et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–2280. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Leahey A. M. Systemic chemotherapy: a pediatric oncology perspective. In: Ramasubramanian A., Shields C. L., editors. Retinoblastoma. New Delhi, India: Jaypee Brothers Medical Publishers; 2012. pp. 81–85. [Google Scholar]
- 38.Rojanaporn D., Chanthanaphak E., Boonyaopas R., et al. Intra-arterial chemotherapy for retinoblastoma: 8-year experience from a tertiary referral institute in Thailand. Asia-Pacific journal of ophthalmology. 2019;8:211–217. doi: 10.22608/APO.2018294. [DOI] [PubMed] [Google Scholar]
- 39.Munier F. L., Gaillard M.-C., Balmer A., et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. British Journal of Ophthalmology. 2012;96(8):1078–1083. doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 40.Kivelä T., Eskelin S., Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118(8):p. 1689. doi: 10.1016/j.ophtha.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Munier F. L., Soliman S., Moulin A. P., Gaillard M.-C., Balmer A., Beck-Popovic M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. British Journal of Ophthalmology. 2012;96(8):1084–1087. doi: 10.1136/bjophthalmol-2011-301016. [DOI] [PubMed] [Google Scholar]
- 42.Francis J. H., Xu X. L., Gobin Y. P., Marr B. P., Brodie S. E., Abramson D. H. Death by water: precautionary water submersion for intravitreal injection of retinoblastoma eyes. The Open Ophthalmology Journal. 2014;8(1):7–11. doi: 10.2174/1874364101408010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honavar S., Singh A. Management of advanced retinoblastoma. Ophthalmology Clinics of North America. 2005;18(1):65–73. doi: 10.1016/j.ohc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Francis J. H., Roosipu N., Levin A. M., et al. Current treatment of bilateral retinoblastoma: the impact of intraarterial and intravitreous chemotherapy. Neoplasia. 2018;20(8):757–763. doi: 10.1016/j.neo.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goolam S., Kana H., Welsh N., Wainwright L., Poole J., Mayet I. A 20-year retrospective review of retinoblastoma at two tertiary academic hospitals in johannesburg, South Africa. Ocular Oncology and Pathology. 2018;4(3):170–175. doi: 10.1159/000481508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berry J. L., Kogachi K., Aziz H. A., et al. Risk of metastasis and orbital recurrence in advanced retinoblastoma eyes treated with systemic chemoreduction versus primary enucleation. Pediatr Blood Cancer. 2017;64(4):1–11. doi: 10.1002/pbc.26270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Nawaiseh I., Jammal H. M., Khader Y. S., Jaradat I., Barham R. Retinoblastoma in Jordan, 2003-2013: ocular survival and associated factors. Ophthalmic Epidemiology. 2014;21(6):406–411. doi: 10.3109/09286586.2014.967781. [DOI] [PubMed] [Google Scholar]
- 48.Luna-Fineman S., Barnoya M., Bonilla M., Fu L., Baez F., Rodríguez-Galindo C. Retinoblastoma in Central America: report from the central American association of pediatric hematology oncology (AHOPCA) Pediatric Blood & Cancer. 2012;58(4):545–550. doi: 10.1002/pbc.23307. [DOI] [PubMed] [Google Scholar]
- 49.Ali A. A. E., Elsheikh S. M. A., Elhaj A., et al. Clinical presentation and outcome of retinoblastoma among children treated at the national cancer institute (NCI) in gezira, Sudan: a single institution experience. Ophthalmic Genetics. 2011;32(2):122–125. doi: 10.3109/13816810.2010.546822. [DOI] [PubMed] [Google Scholar]
- 50.Broaddus E., Topham A., Singh A. D. Survival with retinoblastoma in the USA: 19752004. British Journal of Ophthalmology. 2009;93(1):24–27. doi: 10.1136/bjo.2008.143842. [DOI] [PubMed] [Google Scholar]
- 51.Jamalia R., Sunder R., Alagaratnam J., et al. Retinoblastoma registry report--hospital kuala lumpur experience. Medical Journal Malaysia. 2010;65(Suppl A):128–130. [PubMed] [Google Scholar]
- 52.Shields C. L., Mashayekhi A., Demirci H., et al. Practical approach to management of retinoblastoma. Archives of Ophthalmology. 2004;122(5):729–735. doi: 10.1001/archopht.122.5.729. [DOI] [PubMed] [Google Scholar]
- 53.Butros L. J., Abramson D. H., Dunkel I. J. Delayed diagnosis of retinoblastoma: analysis of degree, cause, and potential consequences. Pediatrics. 2002;109(3):p. E45. doi: 10.1542/peds.109.3.e45. [DOI] [PubMed] [Google Scholar]
- 54.Buckley J. D. The aetiology of cancer in the very young. The British Journal of Cancer. Supplement. 1992;18:S8–S12. [PMC free article] [PubMed] [Google Scholar]
- 55.Abramson D. H., Frank C. M., Susman M., Whalen M. P., Dunkel I. J., Boyd N. W. Presenting signs of retinoblastoma. The Journal of Pediatrics. 1998;132(3):505–508. doi: 10.1016/s0022-3476(98)70028-9. [DOI] [PubMed] [Google Scholar]
- 56.Jubran R. F., Erdreich-Epstein A., Butturini A., Murphree A. L., Villablanca J. G. Approaches to treatment for extraocular retinoblastoma. Journal of Pediatric Hematology/Oncology. 2004;26(1):31–34. doi: 10.1097/00043426-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Shields C. L., Meadows A. T., Shields J. A., et al. Chemoreduction for retinoblastoma may prevent intracranial neuroblastic malignancy (trilateral retinoblastoma) Archives of Ophthalmology. 2001;119(9):1269–1272. doi: 10.1001/archopht.119.9.1269. [DOI] [PubMed] [Google Scholar]
- 58.Munier F. L., Beck-Popovic M., Chantada G. L., et al. Conservative management of retinoblastoma: challenging orthodoxy without compromising the state of metastatic grace. “Alive, with good vision and no comorbidity. Progress in Retinal and Eye Research. 2019;73:p. 100764. doi: 10.1016/j.preteyeres.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 59.De Jong M. C., Kors W. A., de Graaf P., Castelijns J. A., Moll A. C., Kivelä T. The incidence of trilateral retinoblastoma: a systematic review and meta-analysis. American Journal of Ophthalmology. 2015;160(6):1116–1126. doi: 10.1016/j.ajo.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan E. M., Wilson M. W., Billups C. A., et al. Pathologic risk-based adjuvant chemotherapy for unilateral retinoblastoma following enucleation. Journal of Pediatric Hematology/Oncology. 2014;36(6):e335–e340. doi: 10.1097/mph.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Table 1. The detailed characteristics and treatment course of dead patients.
Data Availability Statement
All the data used to support the findings of this study are included within the article and are available from the corresponding author by a reasonable request.


