2.
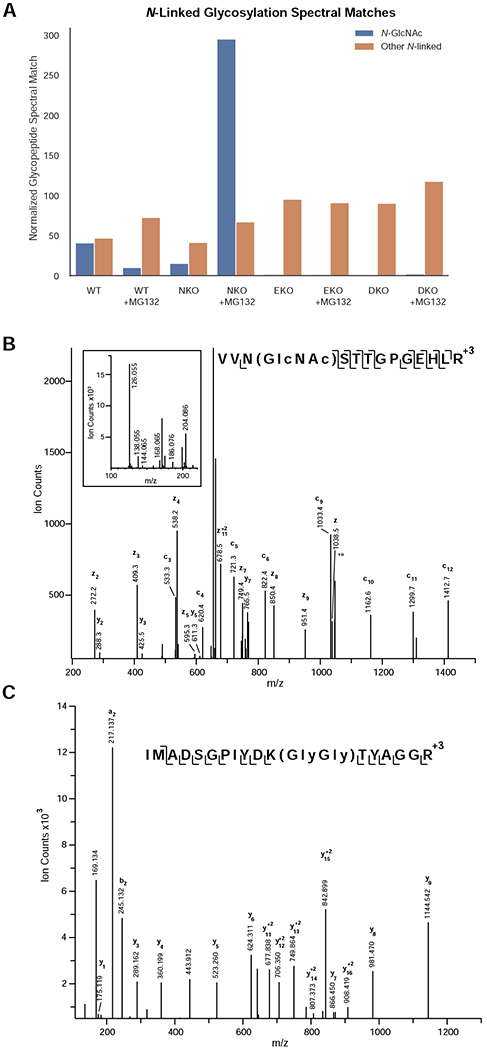
N-GlcNAc proteins are formed by the action of ENGase in the absence of Ngly1. A) Abundance of N-GlcNAc and extended N-glycopeptides in wild type, Ngly1-KO, Engase-KO, and Ngly1/Engase-KO treated with and without proteosomal inhibitor MG132. Peptide spectral matches were normalized to total spectra. Levels of extended N-linked glycopeptide-PSMs were relatively unchanged while the levels of N-GlcNAc modified PSMs were dramatically increased in Ngly1-KO cells upon proteosomal inhibition. B) Annotated ETD MS2 peaklist of N-GlcNAc modified peptide from Thrombospondin-1. C) Annotated HCD MS2 peaklist of K-GlyGly modified peptide from Thrombospondin-1.
