Figure.
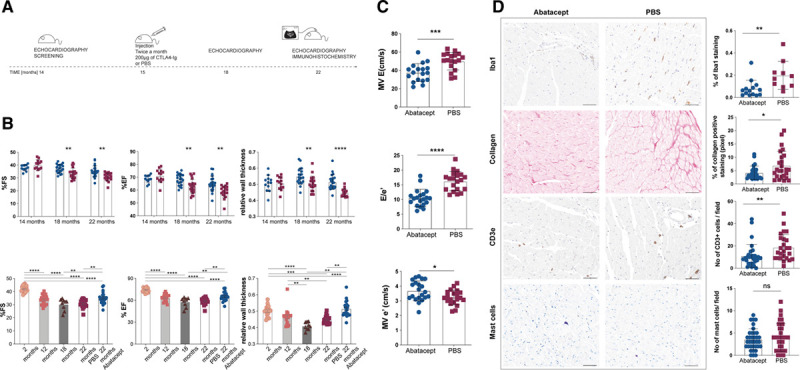
Abatacept treatment significantly improves systolic and diastolic function in old mice. A. Schematic representation of the therapeutic protocol applied to aging mice. C57BL6/J male mice were screened via echocardiography at 14 mo of age, and starting at 15 mo of age received 2×/mo 200 µg abatacept in 100 µL of PBS or 100 µL of PBS via intraperitoneal injection, for further 7 mo. Systolic heart functionality at 18 and 22 mo of age, and diastolic heart functionality at 22 mo of age via echocardiography was recorded and analyzed by a blinded operator. B, Abatacept treatment improves systolic function. %fractional shortening (FS), %ejection fraction (EF), and relative wall thickness (RWT) of 14-, 18- and 22-mo-old C57BL6/J mice treated with abatacept (white column/blue circles) or with PBS (white column/red squares) are plotted as mean±SEM (abatacept-treated n=24, PBS-treated n=20). %FS, %EF and RWT of 2- (white column/orange circles), 12-(light gray column/pink squares), 18-mo-old (dark gray column/burgundy triangles), 22 mo-old treated with PBS (white column/red squares) and 22-mo-old abatacept-treated (white column/blue circles) C57BL6/J male mice are plotted as Scatter plot with bar; columns represent the mean and each dot represents one mouse (2 mo n=18, 12 mo n=19, 18 mo n=9, abatacept-treated n=24, PBS-treated n=20). Full legend and statistical details in the Data Supplement. C, Abatacept treatment improves diastolic function. Echocardiographic analysis of transmitral early peak velocity (E), early diastolic mitral annulus velocity (e′) and E/e′ estimated by transmitral Doppler and tissue Doppler of 22 mo-old C57BL6/J mice treated with abatacept (white column/blue circles) or with PBS (white column/red squares) are plotted as mean±SEM (n=20). Shapiro-Wilk test was performed to confirm normal distribution. Unpaired t test. *P=2.04×10-2; ****P=1.12×10-6. D, Macrophage infiltration, collagen deposition, and T celll infiltration but not mast cell presence is reduced by abatacept treatment. Full legend is given in the Data Supplement.
