Figure 5.
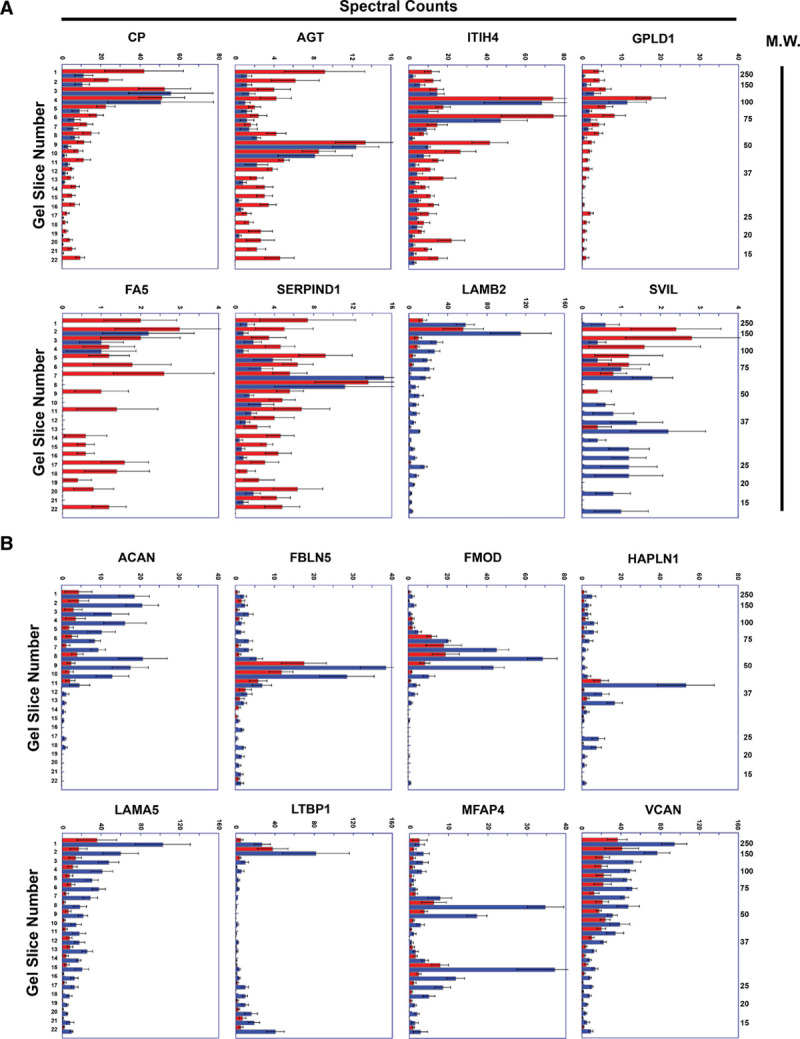
Representative peptographs of extracts of ruptured vs stable human plaque segments.
A and B, Extracts of ruptured (red) and adjacent stable (blue) segments of 5 human carotid plaques were analyzed using the PROTOMAP protocol. The extracts were subjected to SDS-PAGE, and the gels were cut into 22 slices, each corresponding to a molecular weight range. After in-gel trypsin digestion, peptides were extracted, identified by tandem mass spectrometry, and spectral counts were aggregated over all 22 slices. A, Proteins with differential abundance of lower-molecular weight peptides in extracts of ruptured vs. stable segments: ceruloplasmin (CP), angiotensinogen (AGT), ITIH4 (inter-alpha-trypsin inhibitor heavy chain), GPLD1 (phosphatidylinositol-glycan-specific phospholipase D), FA5 (coagulation factor V), SERPIND1 (serpin family D member 1), LAMB2 (laminin subunit beta-2), and SVIL (supervillin). B, ECM (Extracellular matrix) proteins that are significantly less abundant in extracts of ruptured vs stable human plaque segments: ACAN (aggrecan core protein), FBLN5 (fibulin 5), FMOD (fibromodulin), HAPLN (hyaluronan and proteoglycan link protein 1), LAMA5 (laminin subunit alpha 5), MFAP4 (microfibril-associated glycoprotein 4), LTBP1 (latent-transforming growth factor beta-binding protein 1), and VCAN (versican). A and B, Horizontal bars in each peptograph portray the total spectral counts for protein-specific peptides in each of the 22 gel slices (mean±SEM; n=5). Gel-slice number is on the leftward y-axis; molecular weight of the gel slices (in kilodaltons) is on the rightward y-axis.
