Abstract
Background
The predictive value of hemoglobin A1c (HbA1c) levels in non-diabetic patients with myocardial infarction undergoing percutaneous coronary intervention (PCI) is still controversial. This study aimed to evaluate whether HbA1c levels were independently associated with adverse clinical outcomes in non-diabetic patients with coronary artery disease (CAD) who had undergone PCI by performing a meta-analysis of cohort studies.
Methods
This meta-analysis included non-diabetic patients with CAD who had undergone PCI. A systematic search for publications listed in the PubMed, Embase, and Cochrane Library databases from commencement to December 2018 was conducted. Studies evaluating the adverse clinical outcomes according to abnormal HbA1c levels in non-diabetic patients diagnosed with CAD who had undergone PCI were eligible. The primary outcomes were long-term all-cause deaths and long-term major adverse cardiac events, and the secondary outcome was short-term all-cause deaths. The meta-analysis was conducted with RevMan 5.3 and Stata software 14.0. Odds ratios (ORs) were pooled using a random or fixed-effects model, depending on the heterogeneity of the included studies. Sub-group analysis or sensitivity analysis was conducted to explore potential sources of heterogeneity, when necessary.
Results
Six prospective cohort studies involving 10,721 patients met the inclusion criteria. From the pooled analysis, abnormal HbA1c levels were associated with increased risk for long-term all-cause death (OR 1.39, 95% confidence interval [CI] 1.16–1.68, P = 0.001, I2 = 45%). Sub-group analysis suggested that abnormal HbA1c levels between 6.0% and 6.5% predicted higher long-term major adverse cardiac event (including all-cause deaths, non-fatal myocardial infarction, target lesion revascularization, target vessel revascularization, recurrent acute myocardial infarction, heart failure requiring hospitalization, and stent thrombosis) risk (OR 2.05, 95% CI 1.46–2.87, P < 0.001, I2 = 0). Contrarily, elevated HbA1c levels were not associated with increased risk of short-term all-cause death (OR 1.16, 95% CI 0.88–1.54, P = 0.300, I2 = 0).
Conclusions
An abnormal HbA1c level is an independent risk factor for long-term adverse clinical events in non-diabetic patients with CAD after PCI. Strict control of HbA1c levels may improve patient survival. Further studies in different countries and prospective cohort studies with a large sample size are required to verify the association.
Keywords: Acute myocardial infarction, Hemoglobin A1c, Percutaneous coronary intervention, Pre-diabetes
Introduction
Coronary artery disease (CAD) threatens global health and has led to an increase in mortality worldwide.[1–3] In 1977, the first percutaneous coronary intervention (PCI) was performed. Currently, PCI has become one of the most frequently performed therapeutic interventions in acute myocardial infarction cases, resulting in a steady decline in periprocedural adverse events.[4,5] Hemoglobin A1c (HbA1c) level is an indicator of the average blood glucose concentrations over the preceding 2 to 3 months, which is a convenient and well-known biomarker in clinical practice.[6] Since 2010, HbA1c has been recommended by the World Health Organization and American Diabetes Association as a point-of-care test for the diagnosis of diabetes mellitus (≥6.5%).[7] An elevated HbA1c level is associated with an increased risk of major adverse cardiac events (MACEs) in diabetes mellitus patients undergoing PCI.[8] However, to date, the predictive value of HbA1c levels in non-diabetic patients with myocardial infarction undergoing PCI is still controversial.[9–15] Considering the limited information and uncertain effects of HbA1c levels on the prognosis after acute myocardial infarction, we aimed to evaluate whether HbA1c levels were independently associated with the adverse clinical outcomes in non-diabetic patients diagnosed with CAD after PCI by performing a meta-analysis of cohort studies.
Methods
The methods for this meta-analysis are in accordance with the Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting,[16] following a registered protocol on the PROSPERO database (No. CRD42019119603).
Data sources and search strategy
A systematic search of publications listed in the databases (PubMed, Embase, The Cochrane Library) from commencements to December 2018 was conducted using the following search terms: (“Coronary Intervention, Percutaneous” or “Coronary Interventions, Percutaneous” or “Intervention, Percutaneous Coronary” or “Interventions, Percutaneous Coronary” or “Percutaneous Coronary Interventions” or “Percutaneous Coronary Revascularization” or “Coronary Revascularization, Percutaneous” or “Coronary Revascularizations, Percutaneous” or “Percutaneous Coronary Revascularizations” or “Revascularization, Percutaneous Coronary” or “Revascularizations, Percutaneous Coronary”) and (“Hemoglobin A, Glycated” or “Hb A1c” or “HbA1” or “Glycosylated Hemoglobin A” or “Hemoglobin A, Glycosylated” or “Hb A1” or “Glycohemoglobin A” or “Glycated Hemoglobin A1c” or “Hemoglobin A1c, Glycated” or “Glycosylated Hemoglobin A1c” or “Hemoglobin A1c, Glycosylated” or “Glycated Hemoglobins” or “Hemoglobins, Glycated” or “Hemoglobin, Glycosylated” or “Glycosylated Hemoglobin”). All references in the retrieved articles were also scanned to identify other potentially available studies.
Inclusion criteria, data extraction
We included studies published until December 2018 about abnormal HbA1c levels and adverse clinical outcomes in non-diabetic patients diagnosed with CAD undergoing PCI and found that abnormal HbA1c levels were discrepant in different studies included. Therefore, to avoid eliminating studies with important information, we considered that the abnormal HbA1c cut-off levels should be ≥5.7%.
All of the included studies were observational cohort studies. Non-diabetic patients were defined as those without a history of diabetes at admission, who never received anti-diabetic treatment before. The following information was assessed and extracted from the included studies: (1) description of the study's characteristics, including last name of first author, year of publication, country of origin, and number of enrolled patients; (2) description of patient sample characteristics, including age, sex, body mass index, smoking status, hypertension, dyslipidemia, and family history of CAD; (3) time of measuring HbA1c levels; (4) clear inclusion and exclusion criteria; (5) potential selection bias; (6) completeness of follow-up; (7) adverse clinical outcomes in different studies; and (8) adjustment of possible confounders in the multivariate analysis.
Definition of outcomes
The main adverse clinical outcomes assessed in our study were long-term all-cause deaths and long-term MACEs, and the second outcome was short-term all-cause deaths. The definition of long-term was at least 1 year of follow-up, whereas short-term included in-hospital and 30-day follow-up. All-cause deaths, non-fatal myocardial infarction, target lesion revascularization, target vessel revascularization, recurrent acute myocardial infarction, heart failure requiring hospitalization, and stent thrombosis were all considered MACEs.
Quality assessment
The quality of the included studies was assessed using the Newcastle Ottawa scale.[17] This scale awards a maximum of nine stars to each study, and a system was developed to judge a study based on the following three broad perspectives: selection of the study groups (0–4 points), comparability of the groups (0–2 points), and ascertainment of either the exposure or outcome of interest (0–3 points). Quality was categorized as follows: high quality with 6 to 9 stars and sub-optimal quality with 0 to 5 stars.
Statistical analysis
We used RevMan software (version 5.3; Cochrane Collaboration, Oxford, UK) and Stata software (version 14.0; Stata Corporation, College Station, TX, USA) to pool data for all outcomes. P values were considered statistically significant at <0.05. Heterogeneity was assessed using the Cochran χ2 method and the I2 test.[18] When the P value for Cochran method was <0.05 or the I2 was >50%, there was an obvious heterogeneity among those included studies. The random-effects model (DerSimonian and Laird method) for calculating the pooled odds ratio (OR) and 95% confidence interval (CI) was used. When there was no obvious heterogeneity among those included studies, the fixed-effects model was used. A potential publication bias was assessed by visual inspection of the funnel plot; an asymmetric plot suggested a possible publication bias. This study also performed the Egger linear regression test to assess the funnel-plot's asymmetry. Sensitivity analysis was processed by excluding each study in turn. In addition, we performed a sub-group analysis to investigate the association between HbA1c levels and long-term all-cause deaths according to patients from different countries. We also further performed sub-group analyses according to the different definitions of abnormal HbA1c levels.
Results
Characteristics of the studies and publication bias
The selection process of potentially relevant studies is shown in a flow chart [Figure 1]. Of the studies, six with a total of 10,721 patients met the inclusion criteria and were used for this meta-analysis,[19–24] and we found no evidence of publication bias based on the funnel plot or when using the Egger linear regression test (P = 0.407). Baseline characteristics of the six studies and the included patients who were grouped by different HbA1c levels are shown in Tables 1–3.
Figure 1.
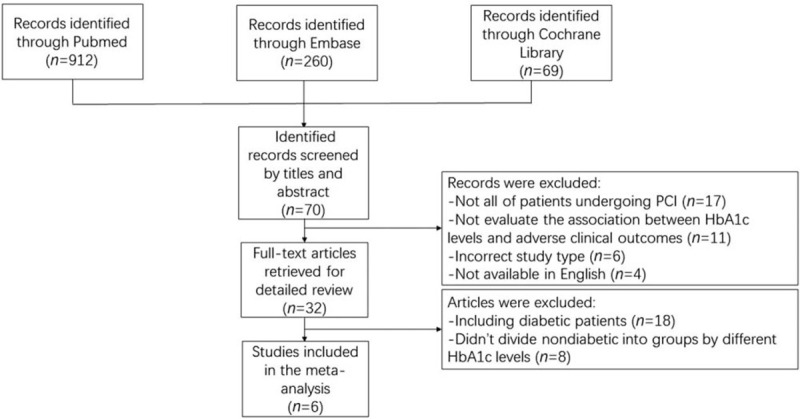
Flow chart of the included observational studies. PCI: Percutaneous coronary intervention; HbA1c: Hemoglobin A1c.
Table 1.
Characteristics of the six studies included in the meta-analysis.
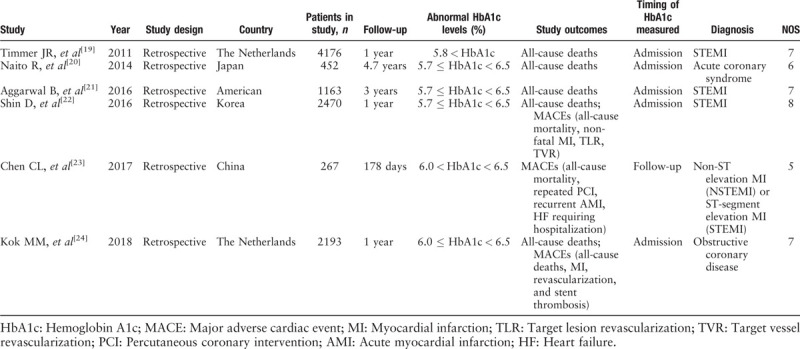
Table 3.
Baseline characteristics of the included patients with normal HbA1c levels.
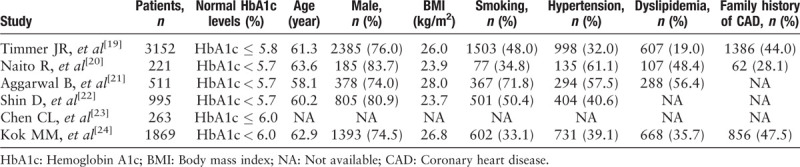
Table 2.
Baseline characteristics of the included patients with abnormal HbA1c levels.
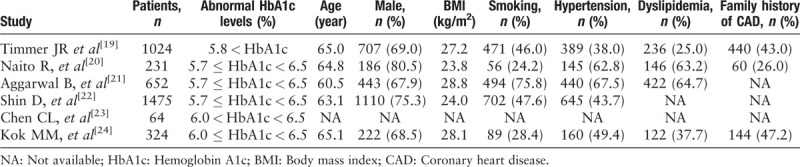
Five of the included six studies reported long-term all-cause deaths,[19–22,24] three studies had long-term MACE outcomes at follow-up,[22–24] and three of the six studies investigated short-term all-cause deaths.[19,22,23] Among the six studies, three studies were from Asia, two from the Netherlands, and one from the United States. Five of the six studies were of high quality, whereas the other was of sub-optimal quality.
Meta analysis of long-term all-cause deaths
Five studies reported that long-term all-cause deaths and abnormal HbA1c levels were associated with increased risk of long-term all-cause deaths (OR 1.39, 95% CI 1.16–1.68, P = 0.001, I2 = 45%) [Figure 2]. There was a moderate statistical heterogeneity among the studies. We also conducted a sensitivity analysis by excluding each study in turn for heterogeneity. There were no significant changes.
Figure 2.
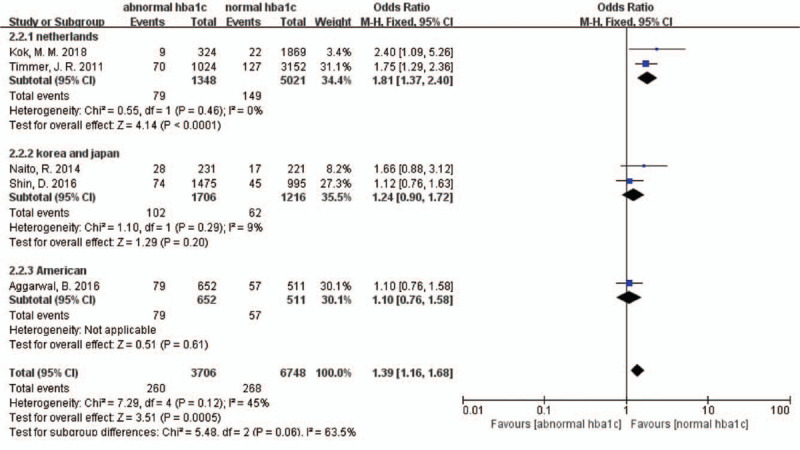
Forest plot for long-term all-cause deaths sub-grouped by different countries. CI: Confidence interval.
In the sub-group analysis according to different countries, in patients from the Netherlands, elevated admission HbA1c levels were associated with an 81% increased risk of death (OR 1.81, 95% CI 1.37–2.40, P < 0.001, I2 = 0), whereas in patients from other countries, increased HbA1c levels were not associated with a significantly higher risk of death (Korea and Japan: OR 1.24, 95% CI 0.90–1.72, P = 0.200, I2 = 9%; USA: OR 1.10, 95% CI 0.76–1.58, P = 0.610) [Figure 2].
Meta-analysis of long-term MACEs
Three studies investigated the association between abnormal HbA1c levels and long-term MACEs. However, abnormal HbA1c levels did not lead to increased long-term MACEs (OR 1.59, 95% CI 1.00–2.56, P = 0.050, I2 = 72%) [Figure 3]. There was a marked heterogeneity in the data. We conducted a sensitivity analysis by excluding each study in turn for heterogeneity. We found that the marked heterogeneity came from the Shin's study and the HbA1c cut-off levels were between 5.7% and 6.5% in their study.
Figure 3.
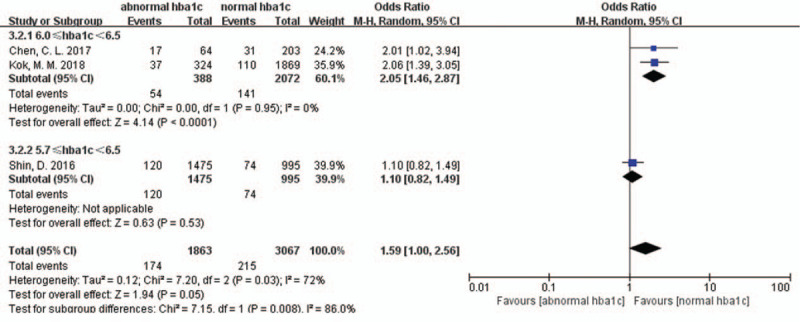
Forest plot for long-term major adverse cardiac events sub-grouped by different definitions of abnormal HbA1c level. CI: Confidence interval; HbA1c: Hemoglobin A1c.
In our sub-group analyses according to the different definitions of abnormal HbA1c levels, it should be noted that an increased long-term MACE ratio was noticed when the abnormal HbA1c cut-off levels were between 6.0% and 6.5% (OR 2.05, 95% CI 1.46–2.87, P < 0.001, I2 = 0) [Figure 3]. The sub-group analytic heterogeneity was very low.
Meta-analysis of short-term all-cause deaths
Studies by Timmer et al, Aggarwal et al, and Shin et al investigated whether the abnormal HbA1c levels in patients at admission were associated with an increase in short-term all-cause deaths.[19,21,22] However, no statistically significant discrepancy was found (OR 1.16, 95% CI 0.88–1.54, P = 0.3, I2 = 0) [Figure 4], and the research had a moderate heterogeneity. We conducted a sensitivity analysis for heterogeneity and do not found apparent changes.
Figure 4.
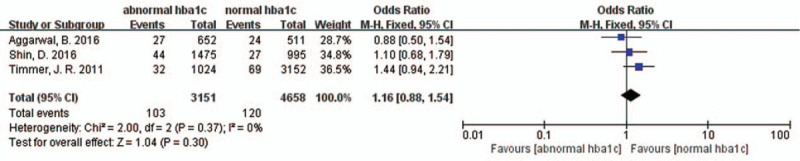
Forest plot for short-term all-cause deaths. CI: Confidence interval.
Discussion
Although some studies were published to assess the association between elevated HbA1c levels and adverse clinical outcomes in CAD patients, no consistent results were reported.[9,10,14,25–27] Thus, we performed this meta-analysis of six cohort studies with a total of 10,721 non-diabetic patients diagnosed with CAD who underwent PCI. To the best of our knowledge, this meta-analysis is among the first to aim at assessing the impact of abnormal HbA1c levels on short-term and long-term adverse clinical events in this patient cohort.
The results of our meta-analysis suggested that abnormal HbA1c levels predicted an increased risk of long-term all-cause death in patients hospitalized with CAD undergoing PCI. Apart from our study, several other studies have also showed that HbA1c levels are potent predictors of long-term deaths.[10,14,27] There are several possible explanations for the adverse impact of abnormal HbA1c levels on long-term all-cause death risk in non-diabetic patients receiving PCI. First, increased HbA1c levels were a measure of poor previous glycemic control, and there was evidence that chronic hyperglycemia can induce vascular endothelial cell damage, with resulting vasomotor dysfunction, excessive extracellular matrix formation, and increased cellular proliferation.[28] Second, Saleem et al's study found that the HbA1c level was an independent factor influencing the severity of CAD, as demonstrated by coronary angiography.[29] Third, an increase in HbA1c levels was clearly associated with adverse baseline characteristics such as a higher cardiovascular risk profile, and this study may partly explain the increase in long-term deaths.[19] However, our sub-group analysis revealed that, in studies involving Asian and American patients, abnormal HbA1c levels are not associated with increased long-term all-cause deaths. According to our meta-analysis, we can suppose that the relationship between HbA1c levels and long-term all-cause deaths may be affected by the differences among countries. More studies on the regional disparity would be needed to confirm this.
The International Expert Committee, appointed by the American Diabetes Association, the European Association for the Study of Diabetes, and the International Diabetes Federation recommended HbA1c levels of 6.0% to 6.4% for the identification of an intermediate risk group, as identification of these individuals provides an opportunity for intervention through lifestyle modification and pharmacological interventions to prevent progression to diabetes,[24,30] and several general population studies have found an increased risk of composite cardiovascular events with abnormal HbA1c levels between 6.0% and 6.5%.[31,32] The findings from our sub-group analysis also show that the included patients with HbA1c levels between 6.0% and 6.5% had higher rates of long-term MACEs.
Admission HbA1c levels had no predictive values for short-term all-cause death outcomes based on our meta-analysis study. One possible explanation was that elevated HbA1c levels result from long-term insulin resistance.[33,34] Besides, non-diabetic patients with elevated HbA1c levels had an increased risk of developing diabetes mellitus, which may require long-term follow-up. CAD patients with newly diagnosed diabetes mellitus had a greater risk for developing adverse outcomes. However, in a short-term follow-up, the ability to detect the difference in all-cause deaths may be limited by the small number of incident diabetes mellitus.[35]
Several limitations of this meta-analysis should be noted. First, the pooled studies differed in terms of the inclusion and exclusion criteria, cut-offs for abnormal HbA1c levels, duration of follow-up, and concomitant treatment. These may partially be the source of heterogeneity. Second, given that all included studies were observational, the possibility of residual confounding by unmeasured factors cannot be eliminated. This provided associative, not causal, evidence and mandates caution when interpreting these results. Third, the definitions of MACEs were inconsistent across the three included studies, thereby leading to different rates of MACE outcomes in discrepant studies.
In conclusion, our meta-analysis found that abnormal HbA1c levels ≥5.7% predicted an increased risk of long-term all-cause deaths in hospitalized non-diabetic patients with CAD who were undergoing PCI. Their HbA1c levels ranged from 6.0% to 6.5%, which was associated with higher rates of long-term MACEs. According to our study, cardiologists can understand the CAD patients’ prognosis better through their HbA1c levels. In real life, even though CAD patients receiving PCI do not diagnose DM, they should control their HbA1c levels strictly. When these patients have lower HbA1c levels, they can have lower incidence rates of adverse clinical outcomes in the future and live longer.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81872708) and Municipal Key Laboratory of Clinical Epidemiology, Beijing 100069, China.
Conflicts of interest
None.
Footnotes
How to cite this article: Li Y, Li XW, Zhang YH, Zhang LM, Wu QQ, Bai ZR, Si J, Zuo XB, Shi N, Li J, Chu X. Prognostic significance of the hemoglobin A1c level in non-diabetic patients undergoing percutaneous coronary intervention: a meta-analysis. Chin Med J 2020;133:2229–2235. doi: 10.1097/CM9.0000000000001029
REFERENCES
- 1.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 2.Si J, Li XW, Wang Y, Zhang YH, Wu QQ, Zhang LM, et al. Relationship between serum homocysteine levels and long-term outcomes in patients with ST-segment elevation myocardial infarction. Chin Med J 2019; 132:1028–1036. doi: 10.1097/cm9.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han YL. De-escalation of anti-platelet therapy in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a narrative review. Chin Med J 2019; 132:197–210. doi: 10.1097/cm9.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 5.Cui KY, Lyu SZ, Zhang M, Song XT, Yuan F, Xu F. Drug-eluting balloon versus new-generation drug-eluting stent for the treatment of in-stent restenosis: an updated systematic review and meta-analysis. Chin Med J 2018; 131:600–607. doi: 10.4103/0366-6999.226073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007; 50:2239–2244. doi: 10.1007/s00125-007-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Wang R, Wang Y, Cai S. Glycosylated hemoglobin levels and clinical outcomes in diabetic patients receiving percutaneous coronary interventions: a meta-analysis of cohort studies. Int J Cardiol 2015; 190:143–147. doi: 10.1016/j.ijcard.2015.04.126. [DOI] [PubMed] [Google Scholar]
- 9.Corpus RA, O’Neill WW, Dixon SR, Timmis GC, Devlin WH. Relation of hemoglobin A1c to rate of major adverse cardiac events in nondiabetic patients undergoing percutaneous coronary revascularization. Am J Cardiol 2003; 92:1282–1286. 10.1016/j.amjcard.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury TA, Lasker SS. Elevated glycated haemoglobin in non-diabetic patients is associated with an increased mortality in myocardial infarction. Postgrad Med J 1998; 74:480–481. 10.1136/pgmj.74.874.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenerz A, Nilsson G, Forberg R, Ohrvik J, Malmberg K, Berne C, et al. Basal glucometabolic status has an impact on long-term prognosis following an acute myocardial infarction in non-diabetic patients. J Intern Med 2003; 254:494–503. 10.1046/j.1365-2796.2003.01221.x. [DOI] [PubMed] [Google Scholar]
- 12.Kragelund C, Snorgaard O, Kober L, Bengtsson B, Ottesen M, Hojberg S, et al. Hyperinsulinaemia is associated with increased long-term mortality following acute myocardial infarction in non-diabetic patients. Eur Heart J 2004; 25:1891–1897. doi: 10.1016/j.ehj.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Meier JJ, Deifuss S, Klamann A, Launhardt V, Schmiegel WH, Nauck MA. Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the Langendreer Myocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA). Diabetes Care 2005; 28:2551–2553. doi: 10.2337/diacare.28.10.2551. [DOI] [PubMed] [Google Scholar]
- 14.Rasoul S, Ottervanger JP, Bilo HJ, Timmer JR, van’t Hof AW, Dambrink JH, et al. Glucose dysregulation in nondiabetic patients with ST-elevation myocardial infarction: acute and chronic glucose dysregulation in STEMI. Neth J Med 2007; 65:95–100. doi: 10.1097/MD.0b013e318045a00e. [PubMed] [Google Scholar]
- 15.Gustafsson I, Kistorp CN, James MK, Faber JO, Dickstein K, Hildebrandt PR. Unrecognized glycometabolic disturbance as measured by hemoglobin A1c is associated with a poor outcome after acute myocardial infarction. Am Heart J 2007; 154:470–476. doi: 10.1016/j.ahj.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Cota GF, de Sousa MR, Fereguetti TO, Rabello A. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013; 7:e2195.doi: 10.1371/journal.pntd.0002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation 2011; 124:704–711. doi: 10.1161/circulationaha.110.985911. [DOI] [PubMed] [Google Scholar]
- 20.Naito R, Miyauchi K, Ogita M, Kasai T, Kawaguchi Y, Tsuboi S, et al. Impact of admission glycemia and glycosylated hemoglobin A1c on long-term clinical outcomes of non-diabetic patients with acute coronary syndrome. J Cardiol 2014; 63:106–111. doi: 10.1016/j.jjcc.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal B, Shah GK, Randhawa M, Ellis SG, Lincoff AM, Menon V. Utility of glycated hemoglobin for assessment of glucose metabolism in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2016; 117:749–753. doi: 10.1016/j.amjcard.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 22.Shin D, Ahn J, Cha KS, Park JS, Oh JH, Lee HW, et al. Impact of initial glycosylated hemoglobin level on cardiovascular outcomes in prediabetic patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis 2016; 27:40–46. doi: 10.1097/MCA.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 23.Chen CL, Yen DHT, Lin CS, Tsai SH, Chen SJ, Sheu WHH, et al. Glycated hemoglobin level is an independent predictor of major adverse cardiac events after nonfatal acute myocardial infarction in nondiabetic patients: a retrospective observational study. Medicine (Baltimore) 2017; 96:e6743.doi: 10.1097/md.0000000000006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kok MM, Von Birgelen C, Sattar N, Zocca P, Löwik MM, Danse PW, et al. Prediabetes and its impact on clinical outcome after coronary intervention in a broad patient population. EuroIntervention 2018; 14:e1049–e1056. doi: 10.4244/EIJ-D-17-01067. [DOI] [PubMed] [Google Scholar]
- 25.Hadjadj S, Coisne D, Mauco G, Ragot S, Duengler F, Sosner P, et al. Prognostic value of admission plasma glucose and HbA in acute myocardial infarction. Diabet Med 2004; 21:305–310. doi: 10.1111/j.1464-5491.2004.01112.x. [DOI] [PubMed] [Google Scholar]
- 26.Cakmak M, Cakmak N, Cetemen S, Tanriverdi H, Enc Y, Teskin O, et al. The value of admission glycosylated hemoglobin level in patients with acute myocardial infarction. Can J Cardiol 2008; 24:375–378. 10.1016/s0828-282x(08)70600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cicek G, Uyarel H, Ergelen M, Ayhan E, Abanonu GB, Eren M, et al. Hemoglobin A1c as a prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis 2011; 22:131–137. doi: 10.1097/MCA.0b013e328342c760. [DOI] [PubMed] [Google Scholar]
- 28.Kassaian SE, Goodarzynejad H, Boroumand MA, Salarifar M, Masoudkabir F, Mohajeri-Tehrani MR, et al. Glycosylated hemoglobin (HbA1c) levels and clinical outcomes in diabetic patients following coronary artery stenting. Cardiovasc Diabetol 2012; 11:82.doi: 10.1186/1475-2840-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleem T, Mohammad KH, Abdel-Fattah MM, Abbasi AH. Association of glycosylated haemoglobin level and diabetes mellitus duration with the severity of coronary artery disease. Diab Vasc Dis Res 2008; 5:184–189. doi: 10.3132/dvdr.2008.030. [DOI] [PubMed] [Google Scholar]
- 30.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren B, Pankow JS, Matsushita K, Punjabi NM, Daya NR, Grams M, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2017; 5:34–42. doi: 10.1016/s2213-8587(16)30321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansilal S, Farkouh ME, Fuster V. Role of insulin resistance and hyperglycemia in the development of atherosclerosis. Am J Cardiol 2007; 99:6b–14b. doi: 10.1016/j.amjcard.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Geng J, Lu W, Hu T, Tao S, Zhang H, Chen J, et al. Subclinical hyperthyroidism increases risk of coronary heart disease events in type 2 diabetes mellitus. Endocrine 2015; 49:557–559. doi: 10.1007/s12020-014-0472-y. [DOI] [PubMed] [Google Scholar]
- 35.Geng J, Zhang Y, Wang B, Xie J, Xu B, Li J. Glycosylated hemoglobin levels and clinical outcomes in nondiabetic patients with coronary artery disease: a meta-analysis. Medicine (Baltimore) 2017; 96:e6784.doi: 10.1097/md.0000000000006784. [DOI] [PMC free article] [PubMed] [Google Scholar]


