Abstract
Steady state erythropoiesis generates new erythrocytes at a constant rate, and it has an enormous productive capacity. This production is balanced by the removal of senescent erythrocytes by macrophages in the spleen and liver. Erythroid homeostasis is highly regulated to maintain sufficient erythrocytes for efficient oxygen delivery to the tissues, while avoiding viscosity problems associated with over-production. However, there are times when this constant production of erythrocytes is inhibited or is inadequate, at these times, erythroid output is increased to compensate for the loss of production. In some cases, increased steady state erythropoiesis can offset the loss of erythrocytes but, in response to inflammation caused by infection or tissue damage, steady state erythropoiesis is inhibited. To maintain homeostasis under these conditions, an alternative stress erythropoiesis pathway is activated. Emerging data suggest that the BMP4 dependent stress erythropoiesis pathway is integrated into the inflammatory response and generates a bolus of new erythrocytes that maintains homeostasis until steady state erythropoiesis can resume. In this perspective, we define the mechanisms that generate new erythrocytes when steady state erythropoiesis is impaired and discuss experimental models to study human stress erythropoiesis.
Steady state erythropoiesis has an enormous capacity to generate new erythrocytes. It is estimated that adult humans produce 2.5 x 106 erythrocytes per second1, 2. However, this production is offset by the turnover of senescent erythrocytes in the spleen and liver3. Production and turnover are finely balanced to maintain oxygen delivery to the tissues while avoiding problems with blood viscosity associated with over-production. Steady state erythroid progenitors are derived from immature megakaryocyte erythroid progenitors and multi-potential myeloid progenitors4-9. They develop in close proximity with macrophages in a specialized niche referred to as an erythroblastic island (EBI)10-13. These structures are conserved between rodents and humans2, 14 Despite the capacity of steady state erythropoiesis to produce erythrocytes, there are times when it is unable maintain erythroid homeostasis. Simple blood loss can lead to increased steady state erythropoiesis, but the situation is more complex when inflammation caused by infection or tissue damage inhibits steady state erythropoiesis. At these times an alternative erythropoiesis pathway is required and the BMP4-dependent stress erythropoiesis pathway predominates (for review see15, 16). BMP4-dependent stress erythropoiesis has a different strategy than steady state erythropoiesis. Instead of constantly producing new erythrocytes, the stress erythron produces a bolus of new erythrocytes to maintain homeostasis until steady state erythropoiesis can resume normal erythroid output17-21. In this perspective, we will outline the characteristics of the BMP4 dependent stress erythropoiesis and discuss the utility of different experimental systems to model human stress erythropoiesis.
Stress erythropoiesis is a stem cell-based tissue regeneration response.
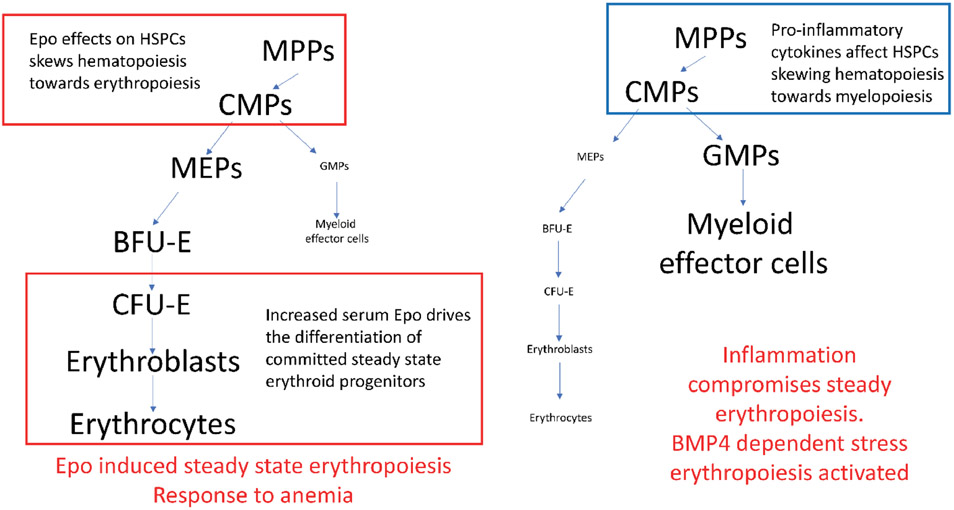
Stress erythropoiesis is a catch all phrase that describes the increase in erythroid output in response to anemic stress. However, this response is more than just increasing steady state erythropoiesis. Early studies in mice analyzed the recovery from phenylhydrazine (PHZ) induced acute hemolytic anemia. These data suggested that bone marrow steady state erythroid progenitors migrated to the spleen where they differentiated in response to the increase serum erythropoietin (Epo) levels induced by tissue hypoxia22-24. Subsequent analysis of mouse strains with mutations that impair stress erythropoiesis showed that this model was incorrect19-21. The increase in erythropoiesis during recovery came from progenitor cells that were distinct from steady state erythroid progenitors and whose development was regulated by many signals that are not involved in the development of steady state erythroid cells. Analysis of flexed-tail (f) mutant mice established a role for BMP4 signaling in the recovery from anemia and for the purposes of this review we will refer to this response as BMP4 dependent stress erythropoiesis19. Before we discuss the mechanisms that regulate this pathway, we need to define the differences between BMP4 dependent stress erythropoiesis and increased steady state erythropoiesis. The BMP4 dependent pathway is best understood in mice and our discussion of the mechanisms that regulate this process will focus on those data. Pro-inflammatory cytokines and alarmins inhibit steady state erythropoiesis and promote myelopoiesis, in order to drive the development of myeloid effector cells25-32. To compensate for the loss of steady state erythropoiesis BMP4 dependent stress erythropoiesis is induced. Unlike steady state erythropoiesis, inflammatory signals act as inducers of this pathway17. In mice, the BMP4 dependent stress erythropoiesis pathway is extramedullary, occurring in the adult spleen and liver33. In response to PHZ induced anemia, a population of stress BFU-E is expanded in the spleen, while at the same time the production of steady state BFU-E in the bone marrow decreases19. This switch in erythroid production from steady state erythropoiesis to BMP4 dependent stress erythropoiesis is a common feature of experimental anemias induced by diverse treatments ranging from PHZ injection to models of sterile inflammation 17-19, 34. In contrast to these treatments, many researchers use treatment with erythropoietin (Epo) to induce stress erythropoiesis, often referred to as Epo-stress. Treatment with Epo does not induce the BMP4 dependent stress erythropoiesis pathway. Although some papers have reported increased erythropoiesis in the spleens of mice treated with Epo, this observation is due to the differentiation of committed late stage erythroid progenitors (CFU-E and erythroblasts) in the spleen and not BMP4 dependent stress erythropoiesis35. In addition, Epo treatment skews steady state hematopoiesis to favor erythropoiesis by increasing the commitment of immature progenitors to the erythroid lineage. In many ways the action of Epo is the opposite of the action of pro-inflammatory cytokines (Figure 1), since Epo increases steady state erythropoiesis, while decreasing steady state myelopoiesis5, 36, 37. The role for Epo in increasing steady state erythropoiesis is also observed in phlebotomy induced anemia. Careful phlebotomy does not induce substantial tissue damage and inflammation and is a weak inducer of the BMP4 dependent pathway.
Figure 1. Schematic of alternate responses to anemia.
Left, steady state erythropoiesis is increased by increased levels of erythropoietin (Epo). Right, Inflammation compromises steady state erythropoiesis, which leads to activation of the BMP4 dependent stress erythropoiesis pathway. MPP-multipotential progenitors, CMP- common myeloid progenitor, MEP-megakaryocyte erythroid progenitor, GMP- granulocyte macrophage progenitor, BFU-e- burst-forming units- erythroid, CFU-E- colony forming units-erythroid. The megakaryocyte pathway is not shown in order to simplify the diagram.
The primary differences between these processes are in the progenitor cells, the signals that regulate their proliferation and commitment to differentiation, and the niche where BMP4 dependent stress erythropoiesis occurs. As described above, in mice, BMP4 dependent stress erythropoiesis is extramedullary. In general, stress erythropoiesis is often referred to as splenic erythropoiesis. This characterization is misleading. Fully grown adult mice (greater than 8-10 weeks old) exhibit little steady state erythropoiesis in the spleen, but in response to inflammatory stress, the spleen is the primary site of BMP4 dependent stress erythropoiesis. However, splenectomized mice are equally capable of responding to anemic stress. In this case, the liver becomes the site of BMP4 dependent stress erythropoiesis. Despite the change in site, liver stress erythropoiesis utilizes the same signals and progenitor cells that are observed in the spleen33. Because of these observations, BMP4 dependent stress erythropoiesis can be thought of as extra-medullary. Although this pathway is conserved in humans, it has not been established that human BMP4 dependent stress erythropoiesis occurs in the spleen. Extramedullary erythropoiesis is observed in many pathological conditions like anemia, malignancy and infection, but the role of the BMP4 dependent pathway in these situations has not been investigated38-43.
The origin and development of immature progenitors is a major difference between steady state and BMP4 dependent stress erythropoiesis. Steady state erythroid progenitors are derived from multipotential progenitors that adopt the erythroid fate. The direct precursor of erythroid progenitor cells is a megakaryocyte-erythroid progenitor (MEP)7, 8. In contrast, stress erythroid progenitors are derived from short-term hematopoietic stem cells (ST-HSCs) that are characterized as CD34+Kit+Sca1+Lineageneg18, 44. In the bone marrow, these cells have the potential to generate all cell lineages, however upon homing to the spleen, signals in the splenic microenvironment commit these cells to the erythroid lineage. The key signals in this commitment step are hedgehog and BMP421. It is most likely Indian hedgehog (Ihh) that regulates this commitment as Sonic hedgehog (Shh) is not expressed in the red pulp of the spleen and Desert hedgehog (Dhh) appears to have a negative effect on stress erythropoiesis45. Hedgehog signaling induces ST-HSCs to express BMP4 and it is the two signals acting together that is required for the specification of the stress erythroid lineage. Mutations in the hedgehog signaling pathway do not affect steady state erythropoiesis. In contrast loss of hedgehog signaling prevents maintenance of the BMP4 dependent pathway. In response to PHZ induced anemia, the BMP4 dependent pathway generates new erythrocytes over the 7-day recovery period. After this initial RBC generation, it takes 21 days before the mouse can respond again to a second anemic challenge. Mutations in hedgehog signaling completely inhibit the regeneration of the pathway preventing subsequent responses to anemia. Furthermore, activation of hedgehog signaling in the bone marrow leads to the development of stress progenitors, suggesting that the compartmentalization of hedgehog signaling restricts stress erythropoiesis to extramedullary sites21.
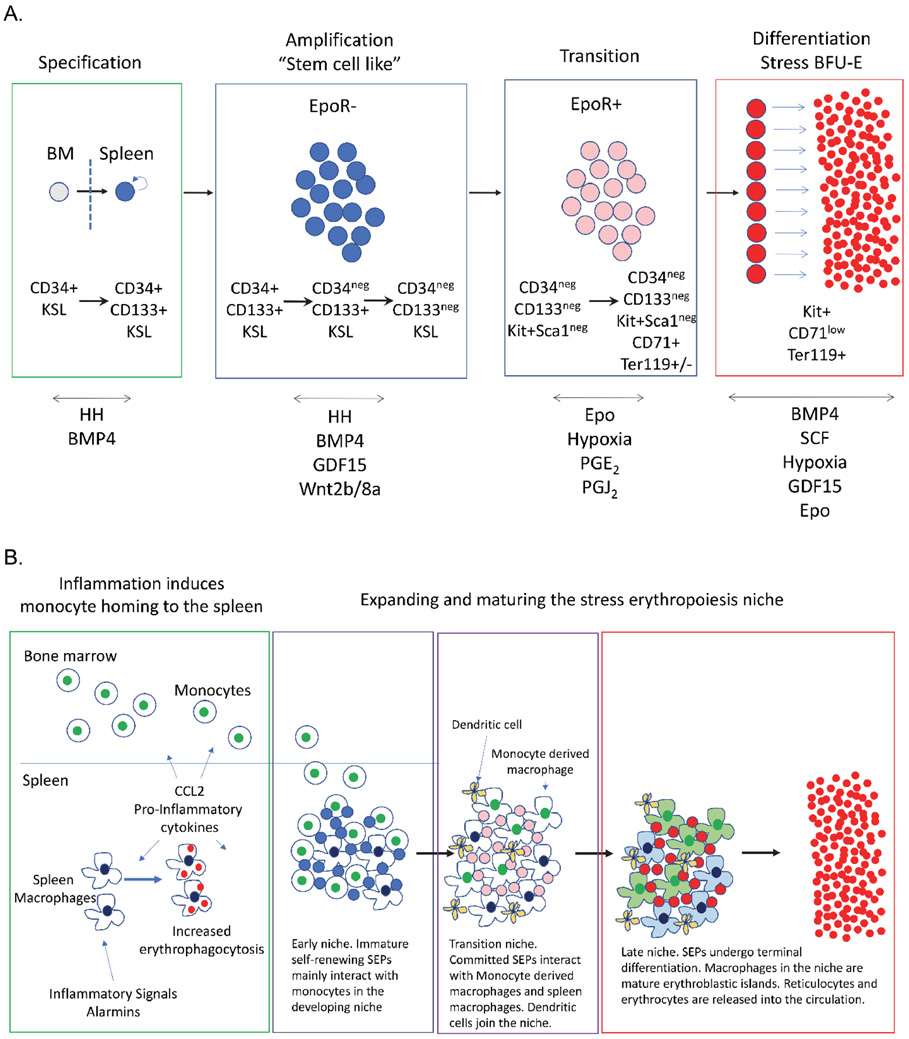
Although BMP4 and Hedgehog signals, restrict the ST-HSCs to the stress erythroid lineage, the immature stress erythroid progenitors (SEPs) maintain stem cell properties. Immature SEPs can be broken down into three populations based on their expression of Kit, Seal, CD34 and CD133 (Figure 2A)18, 44. All three of these populations retain their ability to self-renew and can be serially transplanted18, 44 Following lineage restriction, the SEPs proliferate, but do not differentiate, which generates a transient amplifying population of progenitor cells. This stage in development is characterized by the expression of stem cell markers and a lack of expression of the erythroid program. This amplification step is necessary to generate enough progenitors so that when they differentiate sufficient erythrocytes will be made to maintain homeostasis until steady state erythropoiesis can resume. The signals that drive this expansion include growth and differentiation factor 15 (GDF15) and canonical Wnt signaling46, 47. Mutations in either of these pathways impair the expansion of immature SEPs but have little effect on steady state erythropoiesis47, 48. In addition, Kit receptor and its ligand stem cell factor (SCF) are required for the proliferation of immature SEPs. Mutations in the Kit receptor impair the proliferation of immature SEPs to the point that mice with severe loss of function alleles of Kit lack SEPs in the spleen20. However, unlike mutations GDF15 and the Wnt signaling pathway, mutation of Kit or SCF exhibit a macrocytic anemia demonstrating a need for this signaling pathway in both steady state and BMP4 dependent stress erythropoiesis49. The expansion of immature SEPs is followed by a transition to differentiation. At this stage during their development, the proliferating immature populations of SEPs acquire the ability to differentiate and initiate the erythroid gene expression program. The progenitors lose their ability to self-renew and can no longer be serially transplanted. The signal that drives this transition is Epo, but in this instance Epo is not acting on erythroid progenitor cells (as it would during terminal differentiation), but rather on macrophages in the microenvironment. Epo signaling alters the signals generated by the macrophages, inhibiting the expression of Wnt factors, which promote proliferation and increasing the production of prostaglandins (PGJ2 and PGE2), which promote differentiation44, 46. During this transition, SEPs lose the expression of stem cell markers and start expressing the Epo receptor, which drives terminal differentiation. Other signals that contribute to this transition include corticosteroids that act through the glucocorticoid receptor, which promote the expansion of the population of committed erythroid progenitors. Mutation of the glucocorticoid receptor impairs stress erythropoiesis at this stage. Corticosteroids work in concert with secreted SCF factor to drive the proliferation of committed progenitor cells50-57.
Figure 2. Model of BMP4 dependent stress erythropoiesis.
A. Schematic of the four steps from migration of ST-HSCs into the spleen until terminal differentiation. After specification, the status of Epo receptor expression in the SEPs is indicated. Cell surface markers that identify the populations of SEPs are shown. KSL stands for Kit+Sca1+Lineage negative. The signals known to participate at each stage are indicated below. B. Schematic of the development of the stress erythropoiesis niche in the spleen.
In both steady state and stress erythropoiesis, burst forming units-erythroid (BFU-E) represent the most immature committed erythroid progenitor as defined by colony assays. Stress BFU-E differ from steady state BFU-E in that they can form BFU-E colonies in media containing only Epo. Maximal stress BFU-E production is observed when cells are plated with Epo, BMP4 and SCF at 2% O219, 20, 44. When compared to bone marrow steady state BFU-E, stress BFU-E generate larger colonies, which is consistent with stress BFU-E having a greater capacity to generate new erythrocytes19, 20. Despite these differences, the generation of erythrocytes from CFU-E and erythroblasts in both steady state and the BMP4 dependent pathway require the same genes regulated by key erythroid transcription factors like Gata1, Scl and Klf158. The only difference is that Kit receptor expression is maintained in stress erythroblasts20.
Like all hematopoiesis, BMP4 dependent stress erythropoiesis relies on interactions with the microenvironment. During each of the stages of stress erythropoiesis, SEPs interact with monocytes and macrophages in the microenvironment. Eliminating macrophages in vivo or in vitro blocks the development of SEPs17, 44, 59, 60. The interaction between macrophages and developing erythroid progenitors is a common theme observed in both steady state and BMP4 dependent stress erythropoiesis. These interactions are mediated by adhesion molecules that are expressed by macrophages in both the bone marrow and the spleen. However, mutations revealed that certain adhesion molecules have a greater role in stress erythropoiesis while other function in steady state erythropoiesis61, 62. For example, α4 integrin mutation shows little effect in steady state erythropoiesis but exhibits a delayed recovery from PHZ induced acute anemia61, 62. In contrast, mutation of Maea leads to a defect in steady state erythropoiesis that is compensated by stress erythropoiesis63. The roles of adhesion molecules in regulating BMP4 dependent stress erythropoiesis are complicated by the nature of the adhesion molecules that can function as heterodimers like α4β1 and α5β1 integrins or monomers like Maea and their interactions which can be homotypic like Maea-Maea or heterotypic like α4β1-Vcam1 (for discussion of this complexity see64). In addition to these adhesive interactions, signaling in macrophages plays a key role in regulating SEP development. As described above, Epo signaling in macrophages induces a change in the signals from those that promote proliferation to those that promote differentiation. Macrophages within steady state erythroblastic islands also express the Epo receptor, but the effects of Epo dependent signaling in steady state EBI macrophages is not known and do not appear to increase prostaglandin production as observed in the spleen65.
Not only are the signals from the microenvironment and the interaction between progenitors and macrophages in EBIs different in stress erythropoiesis, the development of the niche is different. Steady state erythropoiesis maintains EBIs in the bone marrow for constant production of erythrocytes2, 66-69, however, the splenic stress erythropoiesis niche is induced by inflammation and develops in concert with SEPs70. The pro-inflammatory signals that inhibit bone marrow erythropoiesis play two roles in stress erythropoiesis. They promote SEP proliferation and the recruitment of monocytes into the spleen to form the niche47, 70. Monocytes mature into macrophages as SEPs proliferate and transition to stress BFU-E, which illustrates the coordinate development of progenitors and the splenic stress erythropoiesis niche (Figure 2B). In addition to monocytes and macrophages, the development of stress erythropoiesis niche also includes other elements. Type 1 conventional dendritic cells and monocytes are derived from a common progenitor71. These dendritic cells are recruited into the niche and play a key role in stress erythropoiesis70. They express SCF and loss of the niche population of dendritic cells impairs recovery from anemia72. Although infection or tissue damage, increases pro-inflammatory cytokine production, which skews hematopoiesis towards the production of myeloid effector cells and inhibits steady state erythropoiesis, these signals lead to increased production and mobilization of monocytes and dendritic cells which subsequently home to the spleen leading to the development of the stress erythroid niche27, 28, 73-75,17, 70. In addition, inflammation induces the production of corticosteroids by the adrenal gland, which act on this niche to promote the maturation monocytes into EBI macrophages76-78. This tight coordination of signals coupled with tissue specific responses to inflammatory signals ensures that the mobilization of immune response is robust without compromising erythroid homeostasis.
The role of inflammation in regulating stress erythropoiesis is similar to other tissue regeneration responses. For example, transient inflammation initiates regeneration following injury in the intestinal epithelium and in skeletal muscle. In these systems, the recruitment of monocytes and macrophages into the sites of injury provide key signals to promote the expansion and differentiation of tissue resident stem cells to repair these tissues79. Like BMP4 dependent stress erythropoiesis, pro-inflammatory microenvironments are associated with the expansion of immature progenitors, while anti-inflammatory or pro-resolving signals are associated with differentiation. This reliance on resolution of inflammatory signals for terminal differentiation provides a basis for the observation that chronic inflammation impairs regeneration79-82. In erythropoiesis, chronic inflammation leads to anemia. Many of these chronic anemias, including sickle cell disease, hemolytic anemia and the anemia of chronic disease (ACD), have underlying inflammatory components that may contribute to the pathology of the anemia83. Hemolysis releases hemoglobin and heme that become pro-inflammatory mediators, while, in the case of ACD, infections and tissue damage induce inflammatory responses that inhibit steady state erythropoiesis83-85. Inflammatory signals also increase hepcidin production leading to the sequestration of iron and act on phagocytes, increasing their turnover of erythrocytes86-88. Resolution of the underlying cause of inflammation is the best way to treat ACD. In mouse models of sterile inflammatory disease, the mice develop anemia. The BMP4 dependent stress erythropoiesis pathway is active in these models and promotes the initial recovery from the anemia, however the mice develop generalized inflammatory disease which leads to a relapsing anemia17. These data highlight a weakness of this regenerative pathway. Unlike the constant production of steady state erythropoiesis, BMP4 dependent stress erythropoiesis makes a bolus of erythrocytes and then must start over to generate a second wave of erythrocytes. Because of this strategy, chronic inflammatory stress presents a problem for the BMP4 dependent stress erythropoiesis pathway. Constant pro-inflammatory signals prevent the transition to differentiation by maintaining a pro-proliferation microenvironment, which limits erythrocyte production and erodes the ability of this pathway to increase erythroid production to maintain homeostasis.
Model systems to study stress erythropoiesis.
The goal of studying stress erythropoiesis in model organisms is to exploit the experimental advantages of these systems in order to understand the process in humans in sufficient detail that we can then develop new therapies for human anemia. The vulnerability of model systems will always be in how well the mechanisms that regulate stress erythropoiesis in a model organism are conserved in human stress erythropoiesis. It is difficult to answer this question because humans stress erythropoiesis is not easily studied. As mentioned above, most of what we know about BMP4 dependent stress erythropoiesis has come from the study of mice. When comparative studies were done, the data from these studies showed that BMP4 dependent stress erythropoiesis was highly conserved between mouse and human. In the section below, we will discuss the data in the murine and rat systems and how they compare to human stress erythropoiesis.
Because of experimental imitations, the study of human erythropoiesis has been limited to studying anemic patients, which are observational data, while the culture of primary erythroid progenitors isolated from patients or generated from CD34+ cells isolated from cord blood, bone marrow or peripheral blood have yielded more mechanistic data. Although cultures of purified CD34+ cells have been useful in studying human steady state erythropoiesis, stress erythropoiesis is more complex and includes interactions between progenitor cells and a complex microenvironment and niche. From the study of murine stress erythropoiesis, we developed a model for BMP4 dependent stress erythropoiesis (Figure 2)19, 20. This model is recapitulated in an in vitro culture system that uses unfractionated bone marrow cells cultured in media containing BMP4, Shh, GDF15, SCF and Epo44. This culture generates a monocyte derived macrophage microenvironment. By manipulating the factors in the media, we can separate the expansion phase from the differentiation phase. During the expansion phase, the media lacks Epo, and the immature SEP populations are generated. These in vitro generated SEPs are transplantable, providing erythroid short-term radioprotection, exhibit self-renewal in vivo, but are erythroid restricted. Addition of Epo to the media results in changes in the microenvironment that promote a transition of SEPs from self-renewing stem cell like progenitors to committed erythroid progenitors. This culture system has been invaluable for the study of stress erythropoiesis and the results obtained in vitro correlate with in vivo models. A major strength of this culture system is that it can be applied to human bone marrow44. Analysis of human bone marrow cultures showed that they required the same growth factors and generated a similar series of SEP populations with the exception that Sca1 was not a marker for the human SEPs. Manipulating these cultures has provided an experimental platform to dissect the BMP4 dependent stress erythropoiesis pathway in humans. Human cultures form the same monocyte derived macrophage stromal layer that supports the proliferation and differentiation of SEPs. The response of macrophages in the niche is conserved between humans and mice. The comparison of in vitro generated human SEPs with previously identified human stress erythroid progenitors isolated from the peripheral blood of anemic patients showed that the in vitro derived SEPs exhibited the characteristics of patient derived progenitors. Human stress erythropoiesis is associated with the expression of fetal hemoglobin (HbF). Gamma (γ) globin, which replaces β-globin in HbF is silenced in adults through the action the BCL11A repressor complex89-91. Healthy adults usually exhibit 1-5% HbF+ cells in circulation92,93. However, in response to anemia and bone marrow transplant, the percentage of HbF+ cells increases, which suggests that stress erythropoiesis may reactivate the fetal erythroid program94, 95. The increase in HbF+ cells is also observed when anemia is induced in non-human primates96. The source of these HbF+ erythrocytes is unclear, but analysis of erythroid progenitors in thalassemia and sickle cell disease patients identified CD34+KIT+ progenitors that also expressed CD235a. These cells when cultured in vitro gave rise to HbF+ erythrocytes97, 98. CD34, KIT and CD235a (mouse Ter119) are markers observed on murine and human BMP4 dependent SEPs. In vitro derived human SEPs express low levels of BCL11a, which leads to high levels of γ globin and HbF. Similarly, murine SEPs do not express Bcl11a and exhibit higher levels of εy and βh1 globin44. The comparison of the properties of human and murine SEPs generated in vitro with SEPs isolated from anemic patients underscores the conservation of the BMP4 dependent stress erythropoiesis in mouse and human.
In vivo analysis of BMP4 dependent stress erythropoiesis in mice has relied primarily on two experimental systems, erythroid short-term radioprotection (STR) after bone marrow transplant and PHZ induced acute hemolytic anemia34. Following transplant, erythroid STR is maintained by stress erythropoiesis, which generates erythrocytes in the first two weeks after transplant, maintaining erythroid homeostasis until donor stem cells can engraft and begin steady state erythropoiesis18, 47. Erythroid STR is a powerful system to analyze the development of SEPs and the stress erythropoiesis niche in the spleen. The role of the BMP4 dependent stress erythropoiesis pathway in generating new erythrocytes after stem cell transplant in human has not been directly addressed. However, analysis of erythropoiesis after transplant showed that patients exhibit a transient increase in HbF cells99, 100. This increase is also observed in non-human primates101. These observations are consistent with our data showing that human SEPs generated in vitro express high levels of γ globin and HbF and suggest that human BMP4 dependent stress erythropoiesis contributes to erythroid homeostasis after transplant.
Historically, the use of PHZ to induce acute hemolytic anemia has been used to test murine mutations for defects in stress erythropoiesis. This protocol allows for the study of proliferation and differentiation of progenitor cells in the spleen and the concurrent development of the niche. Changes in bone marrow erythropoiesis can easily be assessed at the same time. Using this system, we have shown that unlike steady state erythropoiesis which constantly produces new erythrocytes, BMP4 dependent stress erythropoiesis is cyclical. The time from induction of anemia until the pathway can fully respond to a secondary challenge is 28 days17, 21. The mobilization ST-HSCs from the bone marrow and their homing to the spleen is a regulated process. Normal adult mice do not have circulating ST-HSCs in the peripheral blood that can be cultured in vitro to form SEPs. However, following PHZ induced anemia we observe an increase in peripheral blood mononuclear cells (PBMCs) that can generate SEPs when cultured (Figure S1). We see a peak at 60 hours after PHZ, which is a time when the mouse is nearing recovery from anemia. Similarly, normal human donors do not have PBMCs that give rise to SEPs when cultured. In contrast if we cultured PBMCs from sickle cell disease patients, 7 of 10 patients generated stress BFU-E. In each case where we observed stress BFU-E, culturing PBMCs led to the generation of CD34+CD133+Kit+ SEPs (Figure S2). These data further underscore the conservation of BMP4 dependent stress erythropoiesis between humans and mice and show that patients with sickle cell disease mobilize this conserved stress erythropoiesis pathway.
Given its role in the inflammatory response, two other in vivo models have been used to study BMP4 dependent stress erythropoiesis in the context of inflammatory anemia. Injection of heat-kill Brucella abortus (HKBA) induces an inflammatory anemia in approximately 7 days and the mice recover over the next 21 days102, 103. HKBA injection mimics human anemia of inflammation. A second kinetically similar model uses lipopolysaccharide and the β-glucan, zymosan A to induce a generalized chronic inflammatory disease17, 104 Anemia develops in approximately 7 days, which resolves over the next 21 days. This model rapidly induces stress erythropoiesis before the mice exhibit overt anemia. Although the mice initially recover, the mice progress to relapsing chronic anemia17. There are other infection-based models where stress erythropoiesis and anemia have been studied. The cecal ligation and puncture (CLP) model results in a polymicrobial infection leading to anemia and is a model for sepsis105. Similarly, infection with Salmonella leads to splenic stress erythropoiesis106. These models are more complex models of inflammatory anemia induced by infection where the role of the adaptive immune system must be considered.
The analysis of the BMP4 dependent stress erythropoiesis pathway in mice has laid the foundation for the characterization of a conserved pathway in humans, which is supported by the data from human in vitro cultures and the analysis of SEPs in the peripheral blood of patients. Despite these findings, the role of the BMP4 dependent stress erythropoiesis in responding to anemic stress in humans is questioned. Most of the uncertainty comes from the extra-medullary nature of murine stress erythropoiesis. In C57BL/6 mice, the strain where the BMP4 dependent pathway has been most characterized, overt splenomegaly is observed and there is a significant expansion of SEPs in the spleen during the recovery period19. However, depending on the treatment to induce anemia and the strain of mice used in the experiment, the splenomegaly and expansion of SEP populations in the spleen is variable and some strains show little splenomegaly20. In humans, the location of stress erythropoiesis is confounded by the lack of experimental data. Extra-medullary hematopoiesis and erythropoiesis is observed in humans and is associated with pathological conditions like malignancy and anemia. Whether these cases reflect BMP4 dependent stress erythropoiesis is not known. In some cases, responses in the bone marrow can further complicate the interpretation. For example, in hemolytic anemia, bone marrow hypercellularity with an expansion of erythroid progenitors is observed, but splenomegaly is also seen107. It is not known where compensatory erythropoiesis occurs.
In addition to mice, rats have historically been used to study stress erythropoiesis. A paper by Zhang et al. published in Experimental Hematology suggested that rats are a superior model for human stress erythropoiesis108. The authors base that idea on the observation that rats like humans have abundant bone marrow space, which could allow increased bone marrow erythropoiesis in response to anemic stress. In contrast the marrow space in mice is more restricted. The authors show that ACI inbred rats respond to PHZ induced anemia by increasing the percentage of Kit+ and late stage erythroblasts in the bone marrow to a greater extent than in the spleen. The authors did not observe an increase in BMP4 expression in the spleen at the timepoints assayed. The use of inbred strains illustrates both the strength and the weakness of the rodent system. Inbred strains reduce experimental variability, but different inbred strains exhibit distinct responses to anemic stress20. Analysis of the literature shows that the rat response to anemic stress varies between inbred strains. Like C57BL/6 mice, the response of Wistar and Long Evans rats to PHZ induced anemia and Wistar rats to a model of inflammatory anemia induces compensatory erythropoiesis in spleen rather than the bone marrow109, 110,111, 112. Furthermore, Sprague Dawley rats not only increase splenic erythropoiesis in response to PHZ and pregnancy, they also induce BMP4 dependent stress erythropoiesis in a model of lung injury and chronic stress113, 114 We have observed similar strain specific differences in inbred mice33. Despite its use in early studies of erythropoiesis and stress erythropoiesis, the rat has lagged behind mice as an experimental system. The reagents for the analysis of rat hematopoiesis are not well developed. There are fewer mutant strains of rats, although new mutants could be efficiently developed using Crispr/Cas9 genome editing techniques115. Antibodies to cell surface markers that are well correlated with human cell surface markers have not been developed. Despite these weaknesses. the experimental techniques described above for the murine system have been used or could be easily adapted to the rat system. A more informative use of the rat system to study stress erythropoiesis would only add to our knowledge of stress erythropoiesis.
In addition to rodents, other vertebrate systems have contributed to the study of stress erythropoiesis. Non-human primates have been used to study the regulation fetal hemoglobin in response to anemia. These studies showed that responses in baboons mimic human responses to anemia116. Although these studies have played an important pre-clinical role in the development of compounds to reactivate the expression of γ-globin in adults, the cost of these models and the experimental limitations make their routine use unlikely.
Zebrafish as a vertebrate model organism provides a powerful genetic system to study erythropoiesis and stress erythropoiesis. The use of standard genetic screens and chemical genetic screens have identified a number of mutations that impact hematopoiesis and erythropoiesis117, 118. Analysis of these mutants have identified new developmental processes that are highly conserved in vertebrates. Although only a few studies have looked at erythroid regeneration, treatment with PHZ leads to increased erythropoietic activity in the caudal hematopoietic tissue119. Whereas other studies have suggested a role for BMP signaling in erythroid regeneration120. The use of zebrafish to study stress erythropoiesis is promising albeit not yet fully developed.
Conclusions
The study of stress erythropoiesis provides important insight into the mechanisms by which the hematopoietic system compensates for the loss of erythrocytes and erythroid production. New studies show that stress erythropoiesis is integrated into the inflammatory response. A better understanding of these mechanisms will enable us to exploit these pathways and develop new therapeutics to treat anemia. Experimentally, the murine system is the most advanced and shows high conservation with human stress erythropoiesis. However, no single experimental system is perfectly informative, and only the integration of data from all these experimental systems will promote our understanding of human stress erythropoiesis.
Supplementary Material
Highlights.
Stress erythropoiesis is a complex response to anemic stress
Increased steady state erythropoiesis is driven by Epo
Inflammation impairs steady state erythropoiesis and induces BMP4 dependent stress erythropoiesis
BMP4 dependent stress erythropoiesis is highly conserved in mouse and human
Acknowledgments:
Work in the Paulson lab was funded by NIH DK080040, DK119865, HL146528 and by NIFA-USDA Hatch Funds Project number PEN04605 accession number 1010021. Work in the Little lab was funded by CWRU and Cleveland University Hospitals Medical Center. We thank Margherita Cantorna for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Palis J. Primitive and definitive erythropoiesis in mammals. Frontiers in physiology. 2014;5(3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seu KG, Papoin J, Fessler R, et al. Unraveling Macrophage Heterogeneity in Erythroblastic Islands. Frontiers in immunology. 2017;8(1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klei TR, Meinderts SM, van den Berg TK and van Bruggen R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Frontiers in immunology. 2017;8(73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Ramos A, Chapman B, et al. Clonal dynamics of native haematopoiesis. Nature. 2014;514(7522):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tusi BK, Wolock SL, Weinreb C, et al. Population snapshots predict early haematopoietic and erythroid hierarchies. Nature. 2018;555(7694):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch K, Klapproth K, Barile M, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518(7540):542–546. [DOI] [PubMed] [Google Scholar]
- 7.Karamitros D, Stoilova B, Aboukhalil Z, et al. Single-cell analysis reveals the continuum of human lympho-myeloid progenitor cells. Nature immunology. 2018;19(1):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pronk CJ, Rossi DJ, Mansson R, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell stem cell. 2007;1(4):428–442. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Fraticelli AE, Wolock SL, Weinreb CS, et al. Clonal analysis of lineage fate in native haematopoiesis. Nature. 2018;553(7687):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessis M. [Erythroblastic island, functional unity of bone marrow]. Revue d'hematologie. 1958;13(1):8–11. [PubMed] [Google Scholar]
- 11.Chasis JA and Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manwani D and Bieker JJ. The erythroblastic island. Current topics in developmental biology. 2008;82(23–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohandas N and Chasis JA. The erythroid niche: molecular processes occurring within erythroblastic islands. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine. 2010;17(3):110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hom J, Dulmovits BM, Mohandas N and Blanc L. The erythroblastic island as an emerging paradigm in the anemia of inflammation. Immunologic research. 2015;63(1-3):75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulson RF, Ruan B, Hao S and Chen Y. Stress Erythropoiesis is a Key Inflammatory Response. Cells. 2020;9(3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulson RF, Shi L and Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18(3):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett LF, Liao C, Quickel MD, et al. Inflammation induces stress erythropoiesis through heme-dependent activation of SPI-C. Science signaling. 2019,12(598): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harandi OF, Hedge S, Wu DC, McKeone D and Paulson RF. Murine erythroid short-term radioprotection requires a BMP4-dependent, self-renewing population of stress erythroid progenitors. J Clin Invest. 2010;120(12):4507–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenox LE, Perry JM and Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105(7):2741–2748. [DOI] [PubMed] [Google Scholar]
- 20.Perry JM, Harandi OF and Paulson RF. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood. 2007;109(10):4494–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry JM, Harandi OF, Porayette P, et al. Maintenance of the BMP4-dependent stress erythropoiesis pathway in the murine spleen requires hedgehog signaling. Blood. 2009;113(4):911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara H and Ogawa M. Erthropoietic precursors in mice with phenylhydrazine-induced anemia. American journal of hematology. 1976;1(4):453–458. [DOI] [PubMed] [Google Scholar]
- 23.Hara H and Ogawa M. Erythropoietic precursors in murine blood. Experimental hematology. 1977;5(3):161–165. [PubMed] [Google Scholar]
- 24.Hara H and Ogawa M. Erythropoietic precursors in mice under erythropoietic stimulation and suppression. Experimental hematology. 1977;5(2):141–148. [PubMed] [Google Scholar]
- 25.Molica S, Mirabelli R, Molica M, et al. Clinical relevance and treatment of nonautoimmune anemia in chronic lymphocytic leukemia. Cancer management and research. 2011,3(211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadaki HA, Kritikos HD, Valatas V, Boumpas DT and Eliopoulos GD. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood. 2002;100(2):474–482. [DOI] [PubMed] [Google Scholar]
- 27.Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130(15):1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietras EM, Mirantes-Barbeito C, Fong S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nature cell biology. 2016;18(6):607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swann JW, Koneva LA, Regan-Komito D, et al. IL-33 promotes anemia during chronic inflammation by inhibiting differentiation of erythroid progenitors. The Journal of experimental medicine. 2020; 217(9): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valdes-Ferrer SI, Papoin J, Dancho ME, et al. HMGB1 Mediates Anemia of Inflammation in Murine Sepsis Survivors. Molecular medicine. 2016;21(1):951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao W, Koizumi K, Nishio M, et al. Tumor necrosis factor-alpha inhibits generation of glycophorin A+ cells by CD34+ cells. Experimental hematology. 2002;30(11):1238–1247. [DOI] [PubMed] [Google Scholar]
- 32.Zamai L, Secchiero P, Pierpaoli S, et al. TNF-related apoptosis-inducing ligand (TRAIL) as a negative regulator of normal human erythropoiesis. Blood. 2000;95(12):3716–3724. [PubMed] [Google Scholar]
- 33.Lenox LE, Shi L, Hegde S and Paulson RF. Extramedullary erythropoiesis in the adult liver requires BMP-4/Smad5-dependent signaling. Experimental hematology. 2009;37(5):549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett LF, Liao C and Paulson RF. Stress Erythropoiesis Model Systems. Methods in molecular biology. 2018;1698(91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki M, Ohneda K, Hosoya-Ohmura S, et al. Real-time monitoring of stress erythropoiesis in vivo using Gata1 and beta-globin LCR luciferase transgenic mice. Blood. 2006;108(2):726–733. [DOI] [PubMed] [Google Scholar]
- 36.Grover A, Mancini E, Moore S, et al. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. The Journal of experimental medicine. 2014;211(2):181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh RP, Grinenko T, Ramasz B, et al. Hematopoietic Stem Cells but Not Multipotent Progenitors Drive Erythropoiesis during Chronic Erythroid Stress in EPO Transgenic Mice. Stem cell reports. 2018;10(6):1908–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asadov C, Alimirzoeva Z, Mammadova T, et al. beta-Thalassemia intermedia: a comprehensive overview and novel approaches. International journal of hematology. 2018;108(1):5–21. [DOI] [PubMed] [Google Scholar]
- 39.Beguin Y, Fillet G, Bury J and Fairon Y. Ferrokinetic study of splenic erythropoiesis: relationships among clinical diagnosis, myelofibrosis, splenomegaly, and extramedullary erythropoiesis. American journal of hematology. 1989;32(2):123–128. [DOI] [PubMed] [Google Scholar]
- 40.Oikonomidou PR and Rivella S. What can we learn from ineffective erythropoiesis in thalassemia? Blood reviews. 2018;32(2):130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orphanidou-Vlachou E, Tziakouri-Shiakalli C and Georgiades CS. Extramedullary hemopoiesis. Seminars in ultrasound, CT, and MR. 2014;35(3):255–262. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Miwa Y, Abe-Suzuki S, et al. Extramedullary hematopoiesis: Elucidating the function of the hematopoietic stem cell niche (Review). Molecular medicine reports. 2016;13(1):587–591. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Chen D, Long H and Zhu B. The mechanisms of pathological extramedullary hematopoiesis in diseases. Cellular and molecular life sciences : CMLS. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang J, Wu DC, Chen Y and Paulson RF. In vitro culture of stress erythroid progenitors identifies distinct progenitor populations and analogous human progenitors. Blood. 2015;125(11):1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau CI, Outram SV, Saldana JI, et al. Regulation of murine normal and stress-induced erythropoiesis by Desert Hedgehog. Blood. 2012;119(20):4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Xiang J, Qian F, et al. Epo receptor signaling in macrophages alters the splenic niche to promote erythroid differentiation. Blood. 2020;136(2):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao S, Xiang J, Wu DC, et al. Gdf15 regulates murine stress erythroid progenitor proliferation and the development of the stress erythropoiesis niche. Blood advances. 2019;3(14):2205–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cobas M, Wilson A, Ernst B, et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. The Journal of experimental medicine. 2004;199(2):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell ES. Hereditary anemias of the mouse: a review for geneticists. Advances in genetics. 1979;20(357–459. [PubMed] [Google Scholar]
- 50.Bauer A, Tronche F, Wessely O, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes & development. 1999;13(22):2996–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganguli G, Back J, Sengupta S and Wasylyk B. The p53 tumour suppressor inhibits glucocorticoid-induced proliferation of erythroid progenitors. EMBO reports. 2002;3(6):569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolbus A, Blazquez-Domingo M, Carotta S, et al. Cooperative signaling between cytokine receptors and the glucocorticoid receptor in the expansion of erythroid progenitors: molecular analysis by expression profiling. Blood. 2003;102(9):3136–3146. [DOI] [PubMed] [Google Scholar]
- 53.Lee HY, Gao X, Barrasa MI, et al. PPAR-alpha and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature. 2015;522(7557):474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varricchio L, Tirelli V, Masselli E, et al. The expression of the glucocorticoid receptor in human erythroblasts is uniquely regulated by KIT ligand: implications for stress erythropoiesis. Stem cells and development. 2012;21(15):2852–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Lindern M, Zauner W, Mellitzer G, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94(2):550–559. [PubMed] [Google Scholar]
- 56.Wessely O, Deiner EM, Beug H and von Lindern M. The glucocorticoid receptor is a key regulator of the decision between self-renewal and differentiation in erythroid progenitors. The EMBO journal. 1997;16(2):267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Prak L, Rayon-Estrada V, et al. ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature. 2013;499(7456):92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crispino JD and Weiss MJ. Erythro-megakaryocytic transcription factors associated with hereditary anemia. Blood. 2014;123(20):3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chow A, Huggins M, Ahmed J, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature medicine. 2013;19(4):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramos P, Casu C, Gardenghi S, et al. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nature medicine. 2013;19(4):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulyanova T, Jiang Y, Padilla S, Nakamoto B and Papayannopoulou T. Combinatorial and distinct roles of alpha(5) and alpha(4) integrins in stress erythropoiesis in mice. Blood. 2011;117(3):975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ulyanova T, Padilla SM and Papayannopoulou T. Stage-specific functional roles of integrins in murine erythropoiesis. Experimental hematology. 2014;42(5):404–409 e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei Q, Boulais PE, Zhang D, et al. Maea expressed by macrophages, but not erythroblasts, maintains postnatal murine bone marrow erythroblastic islands. Blood. 2019;133(11):1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ulyanova T, Georgolopoulos G and Papayannopoulou T. Reappraising the role of alpha5 integrin and the microenvironmental support in stress erythropoiesis. Experimental hematology. 2020,81(16–31 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Wang Y, Zhao H, et al. Identification and transcriptome analysis of erythroblastic island macrophages. Blood. 2019;134(5):480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobsen RN, Forristal CE, Raggatt LJ, et al. Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80(+)VCAM1(+)CD169(+)ER-HR3(+)Ly6G(+) erythroid island macrophages in the mouse. Experimental hematology. 2014;42(7):547–561 e544. [DOI] [PubMed] [Google Scholar]
- 67.Jacobsen RN, Nowlan B, Brunck ME, et al. Fms-like tyrosine kinase 3 (Flt3) ligand depletes erythroid island macrophages and blocks medullar erythropoiesis in the mouse. Experimental hematology. 2016;44(3):207–212 e204. [DOI] [PubMed] [Google Scholar]
- 68.Jacobsen RN, Perkins AC and Levesque JP. Macrophages and regulation of erythropoiesis. Current opinion in hematology. 2015;22(3):212–219. [DOI] [PubMed] [Google Scholar]
- 69.Kaur S, Raggatt LJ, Millard SM, et al. Self-repopulating recipient bone marrow resident macrophages promote long-term hematopoietic stem cell engraftment. Blood. 2018;132(7):735–749. [DOI] [PubMed] [Google Scholar]
- 70.Liao C, Prabhu KS and Paulson RF. Monocyte-derived macrophages expand the murine stress erythropoietic niche during the recovery from anemia. Blood. 2018;132(24):2580–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merad M, Sathe P, Helft J, Miller J and Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual review of immunology. 2013;31(563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim TS, Hanak M, Trampont PC and Braciale TJ. Stress-associated erythropoiesis initiation is regulated by type 1 conventional dendritic cells. J Clin Invest. 2015;125(10):3965–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boldin MP, Taganov KD, Rao DS, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. The Journal of experimental medicine. 2011;208(6):1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oduro KA Jr., Liu F, Tan Q, et al. Myeloid skewing in murine autoimmune arthritis occurs in hematopoietic stem and primitive progenitor cells. Blood. 2012;120(11):2203–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao JL, Ma C, O'Connell RM, et al. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell stem cell. 2014;14(4):445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Falchi M, Varricchio L, Martelli F, et al. Dexamethasone targeted directly to macrophages induces macrophage niches that promote erythroid expansion. Haematologica. 2015;100(2):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heideveld E, Hampton-O'Neil LA, Cross SJ, et al. Glucocorticoids induce differentiation of monocytes towards macrophages that share functional and phenotypical aspects with erythroblastic island macrophages. Haematologica. 2018;103(3):395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heideveld E, Masiello F, Marra M, et al. CD14+ cells from peripheral blood positively regulate hematopoietic stem and progenitor cell survival resulting in increased erythroid yield. Haematologica. 2015;100(11):1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nature reviews. Immunology. 2017;17(3):165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asfaha S. Intestinal stem cells and inflammation. Current opinion in pharmacology. 2015;25(62–66. [DOI] [PubMed] [Google Scholar]
- 81.Giannakis N, Sansbury BE, Patsalos A, et al. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nature immunology. 2019;20(5):626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rathinam VAK and Chan FK. Inflammasome, Inflammation, and Tissue Homeostasis. Trends in molecular medicine. 2018;24(3):304–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weiss G, Ganz T and Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Canesin G, Hejazi SM, Swanson KD and Wegiel B. Heme-Derived Metabolic Signals Dictate Immune Responses. Frontiers in immunology. 2020;11(66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nader E, Romana M and Connes P. The Red Blood Cell-Inflammation Vicious Circle in Sickle Cell Disease. Frontiers in immunology. 2020,11(454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cassat JE and Skaar EP. Iron in infection and immunity. Cell host & microbe. 2013;13(5):509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Libregts SF, Gutierrez L, de Bruin AM, et al. Chronic IFN-gamma production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118(9):2578–2588. [DOI] [PubMed] [Google Scholar]
- 88.Soares MP and Weiss G. The Iron age of host-microbe interactions. EMBO reports. 2015;16(11):1482–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu N, Hargreaves VV, Zhu Q, et al. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell. 2018;173(2):430–442 e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Menzel S, Garner C, Gut I, et al. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nature genetics. 2007;39(10):1197–1199. [DOI] [PubMed] [Google Scholar]
- 91.Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322(5909):1839–1842. [DOI] [PubMed] [Google Scholar]
- 92.Diepstraten ST and Hart AH. Modelling human haemoglobin switching. Blood reviews. 2019;33(11–23. [DOI] [PubMed] [Google Scholar]
- 93.Vinjamur DS, Bauer DE and Orkin SH. Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. British journal of haematology. 2018;180(5):630–643. [DOI] [PubMed] [Google Scholar]
- 94.Alter BP, Rappeport JM, Huisman TH, Schroeder WA and Nathan DG. Fetal erythropoiesis following bone marrow transplantation. Blood. 1976;48(6):843–853. [PubMed] [Google Scholar]
- 95.Alter BP, Rosenberg PS, Day T, et al. Genetic regulation of fetal haemoglobin in inherited bone marrow failure syndromes. British journal of haematology. 2013;162(4):542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeSimone J, Biel SI and Heller P. Stimulation of fetal hemoglobin synthesis in baboons by hemolysis and hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 1978;75(6):2937–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luck L, Zeng L, Hiti AL, Weinberg KI and Malik P. Human CD34(+) and CD34(+)CD38(−) hematopoietic progenitors in sickle cell disease differ phenotypically and functionally from normal and suggest distinct subpopulations that generate F cells. Experimental hematology. 2004;32(5):483–493. [DOI] [PubMed] [Google Scholar]
- 98.Mathias LA, Fisher TC, Zeng L, et al. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Experimental hematology. 2000;28(12):1343–1353. [DOI] [PubMed] [Google Scholar]
- 99.Meletis J, Papavasiliou S, Yataganas X, et al. 'Fetal' erythropoiesis following bone marrow transplantation as estimated by the number of F cells in the peripheral blood. Bone marrow transplantation. 1994;14(5):737–740. [PubMed] [Google Scholar]
- 100.Weinberg RS, Schofield JM, Lenes AL, Brochstein J and Alter BP. Adult 'fetal-like' erythropoiesis characterizes recovery from bone marrow transplantation. British journal of haematology. 1986;63(3):415–424. [DOI] [PubMed] [Google Scholar]
- 101.Humbert O, Peterson CW, Norgaard ZK, Radtke S and Kiem HP. A Nonhuman Primate Transplantation Model to Evaluate Hematopoietic Stem Cell Gene Editing Strategies for beta-Hemoglobinopathies. Molecular therapy. Methods & clinical development. 2018;8(75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gardenghi S, Renaud TM, Meloni A, et al. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood. 2014;123(8):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim A, Fung E, Parikh SG, et al. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123(8):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Millot S, Andrieu V, Letteron P, et al. Erythropoietin stimulates spleen BMP4-dependent stress erythropoiesis and partially corrects anemia in a mouse model of generalized inflammation. Blood. 2010;116(26):6072–6081. [DOI] [PubMed] [Google Scholar]
- 105.Mishra SK and Choudhury S. Experimental Protocol for Cecal Ligation and Puncture Model of Polymicrobial Sepsis and Assessment of Vascular Functions in Mice. Methods in molecular biology. 2018;1717(161–187. [DOI] [PubMed] [Google Scholar]
- 106.Jackson A, Nanton MR, O'Donnell H, Akue AD and McSorley SJ. Innate immune activation during Salmonella infection initiates extramedullary erythropoiesis and splenomegaly. Journal of immunology. 2010;185(10):6198–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tabbara IA. Hemolytic anemias. Diagnosis and management. The Medical clinics of North America. 1992;76(3):649–668. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Liu Y, Han X, et al. Rats provide a superior model of human stress erythropoiesis. Experimental hematology. 2019;78(21–34 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mazur A. Metabolism of the stimulated rat spleen. I. Ferrochelatase activity as an index of tissue erythropoiesis. J Clin Invest. 1968;47(10):2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petakov M, Biljanovic-Paunovic L, Jovcic G, et al. The influence of acute sterile inflammation on erythropoiesis in rats. Experimental hematology. 1998;26(3):222–227. [PubMed] [Google Scholar]
- 111.Carmichael RD, Orlic D, Lutton JD and Gordon AS. Effects of anemia and hypertransfusion on neonatal marrow and splenic erythrocytic colony-forming units in vitro. Stem cells. 1982;1(3):165–179. [PubMed] [Google Scholar]
- 112.Orlic D, Wu JM, Carmichael RD, et al. Increased erythropoiesis and 2'5'-A polymerase activity in the marrow and spleen of phenylhydrazine-injected rats. Experimental hematology. 1982;10(5):478–485. [PubMed] [Google Scholar]
- 113.Alamo IG, Kannan KB, Loftus TJ, et al. Severe trauma and chronic stress activates extramedullary erythropoiesis. The journal of trauma and acute care surgery. 2017;83(1):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mattsson R, Mattsson A and Lindahl-Kiessling K. Anemia causes erythropoiesis and increased antibody synthesis in the spleen of the pregnant mouse. Developmental and comparative immunology. 1984;8(1):169–178. [DOI] [PubMed] [Google Scholar]
- 115.Smalley E. CRISPR mouse model boom, rat model renaissance. Nature biotechnology. 2016;34(9):893–894. [DOI] [PubMed] [Google Scholar]
- 116.Lavelle D, DeSimone J and Heller P. Fetal hemoglobin reactivation in baboon and man: a short perspective. American journal of hematology. 1993;42(1):91–95. [DOI] [PubMed] [Google Scholar]
- 117.Avagyan S and Zon LI. Fish to Learn: Insights into Blood Development and Blood Disorders from Zebrafish Hematopoiesis. Human gene therapy. 2016;27(4):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Konantz M, Schurch C, Hanns P, et al. Modeling hematopoietic disorders in zebrafish. Disease models & mechanisms. 2019;12(9): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lenard A, Alghisi E, Daff H, et al. Using zebrafish to model erythroid lineage toxicity and regeneration. Haematologica. 2016;101(5):e164–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McReynolds LJ, Tucker J, Mullins MC and Evans T. Regulation of hematopoiesis by the BMP signaling pathway in adult zebrafish. Experimental hematology. 2008;36(12):1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.