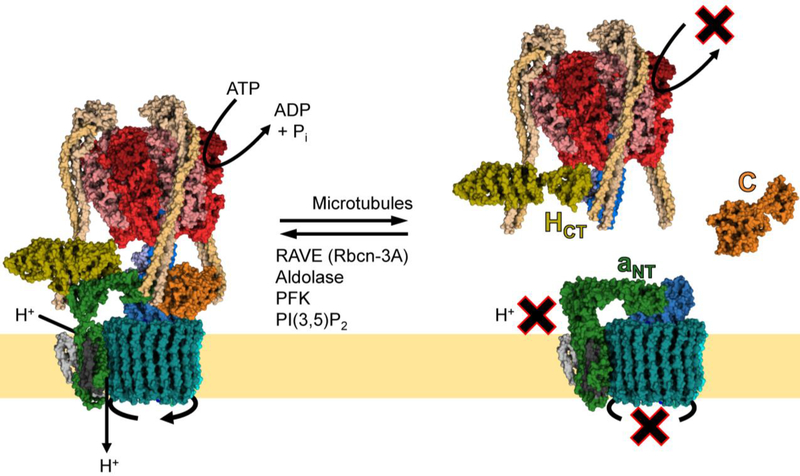
Fig. 2.
Regulated assembly of the V-ATPase.
An important form of V-ATPase regulation is the reversible dissociation of the V1 and V0 domains. In the disassembled state, free V1 cannot hydrolyze ATP, and free V0 cannot conduct protons. In free V1, HCT undergoes a large conformational shift, bringing it into contact with subunit F of the central stalk (PDB ID: 5D80) [45]. In free V0, the N-terminus of Vph1p or Stv1p (aNT) contacts subunit d of the central stalk (PDB ID: 6O7U) [46]. The only subunit that is released from either domain upon disassembly is subunit C. The process of reversible dissociation is rapid, does not require new protein synthesis, and occurs in response to diverse stimuli (see Table 1). In yeast, disassembly requires an intact microtubule network, while reassembly requires the RAVE complex, of which Rav1p is homologous to Rbcn-3A in higher eukaryotes. Other assembly factors common to yeast and higher eukaryotes include the glycolytic enzymes aldolase and phosphofructokinase (PFK). In yeast, PI(3,5)P2 binds to Vph1p and promotes assembly of Vph1p-containing V-ATPase complexes.