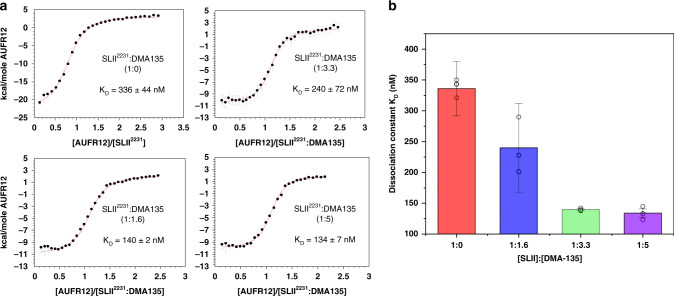
Fig. 6. DMA-135 allosterically stabilizes a AUF1-SLII-(DMA-135) ternary complex.
a Calorimetric titrations of AUF1-RRM1,2 to SLII or to SLII:DMA-135 complex at different molar ratios reveal a single transition in the binding isotherm characterized by an enthalpically driven molecular recognition event. All data were processed and analyzed using Affinimeter25. The processed thermograms were fitted to a 1:1 stoichiometric binding model, represented by the red lines. Values for dissociation constants (KD) and corresponding standard deviation are reported for each titration. The experiments were performed in 10 mm K2HPO4, 20 mm KCl, 0.5 mm EDTA, and 4 mm BME (pH 6.5) buffer at 298 K. b Graphical representation of the dissociation constants for the AUF1-RRM1,2-SLII complex as a function of DMA-135 concentration. Reported KD values and corresponding standard deviations are from n = 3 experimental replicates. Data represented as mean ± SD of triplicate experiments. Uncertainties in individual binding isotherms are calculated using Affinimeter and are determined by considering the noise and quality of raw data and the dispersion of the integrated signal as a function of concentration.