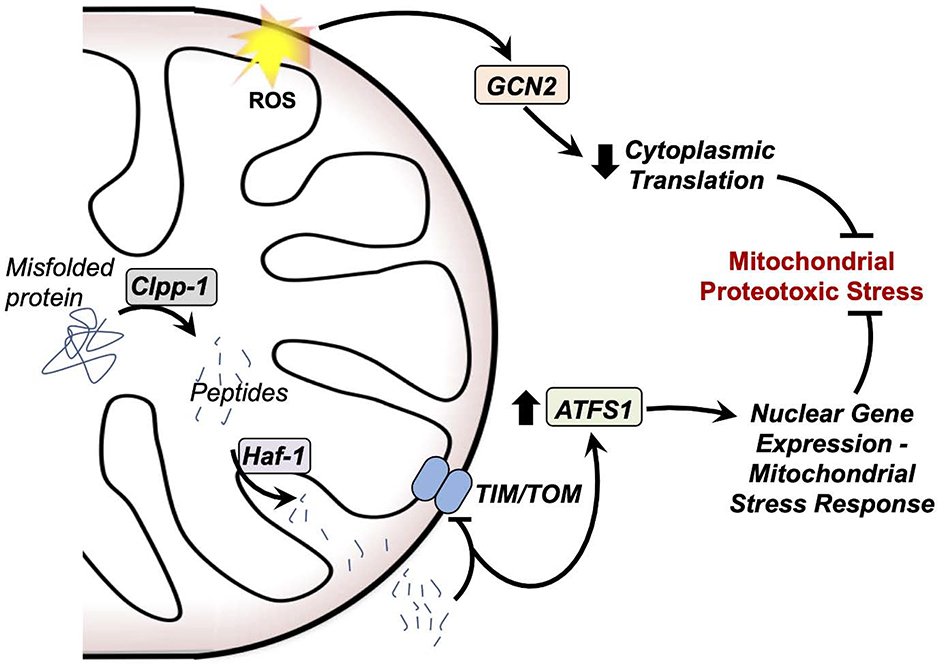
Figure 5 – Mitochondrial unfolded protein responses.
Mitochondria detect and respond to proteotoxic stress through at least two distinct pathways. In one pathway, mitochondrial ROS activate the kinase GCN2, which reduces cytoplasmic translation through inhibition of the eukaryotic transcription initiation factor. In another, misfolded proteins in the mitochondria are cleaved by mitochondrial proteases, including Clpp-1, and exported through the peptide transporter Haf-1. These peptides inhibit the TIM and TOM transport machinery, leading to a cytoplasmic accumulation of the transcription factor ATFS1. ATFS1 upregulates expression of proteins involved in the mtUPR. Together, these two pathways reduce proteotoxic stress at the mitochondria.