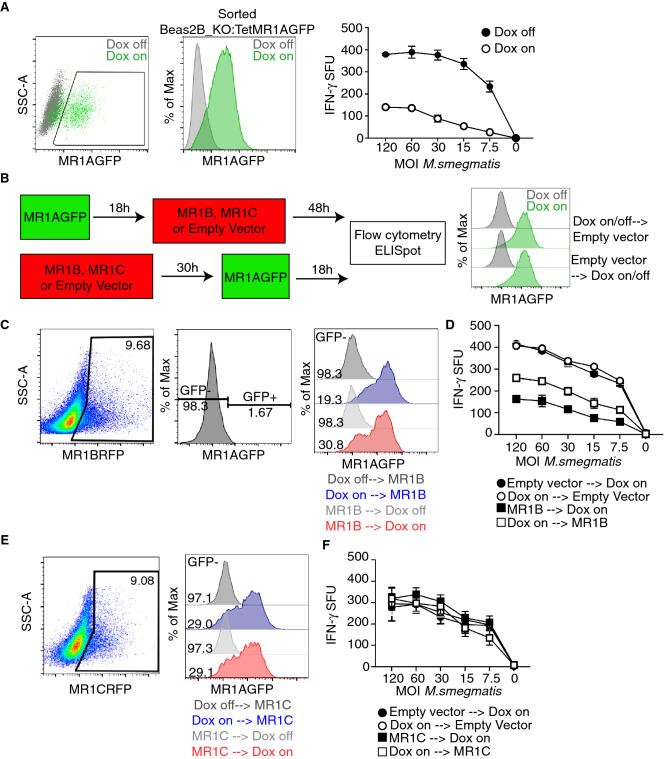
Figure 5.
Prior MR1B expression decreases the abundance of MR1A protein. (A) Beas2B:MR1_KO cells were transduced with a lentivirus encoding a doxycycline-inducible MR1AGFP and sorted based on MR1AGFP expression following overnight administration of doxycycline. Beas2B_MR1KO_TetMR1AGFP cells were treated with doxycycline overnight and infected with M.smegmatis at a dose response of MOI for 1 h, which is indicated on x-axis. Infected cells were used as antigen presenting cells in an ELISpot assay as described earlier to stimulate MAIT production of IFN-γ. Data are representative of at least 3 independent experiments performed in duplicate with similar results. Error bars represent mean and standard deviation from the mean of one experiment. (B) Schematic of experiments to study the timing of MR1A expression with MR1B or MR1C. Briefly, doxycycline was added to Beas2B_MR1KO_TetMR1AGFP cells 18 h prior to or following the transfection with MR1BRFP, MR1CRFP or a pCI empty vector. The timing of transient transfection with MR1BRFP was fixed at 48 h for both conditions to ensure consistency with prior experiments. Doxycycline to induce expression of MR1A was administered for 18 h prior to transient transfection with pCI_MR1BRFP or pCI_MR1CRFP (top), OR ELISpot or flow cytometry to measure function or expression of MR1AGFP. MR1AGFP expression pre and post transfection with a pCI empty vector was measured by flow cytometry (right). (C) Transfected cells were assessed by flow cytometry, gated on MR1B expression using RFP (left), and then assessed for GFP expression, which was based on a dox-off control (second). Overlay histograms of MR1AGFP expression following MR1B (third) transfection as described in Fig. 6B with gMFI of MR1AGFP-negative condition reported. Doxycycline off transfection conditions are included as a control. Data are representative of at least 3 independent experiments with similar results. (D) ELISpot of cells transfected according to (B) and used as antigen presenting cells following infection with M.smegmatis at the indicated MOI (x-axis). Cells were incubated with a MAIT clone and IFN-γ production was measured. Data are pooled from 3 independent experiments performed in duplicate and error bars represent mean and standard deviation of replicates (n = 6). (E) Beas2B:MR1_KO cells were transfected with a plasmid expressing MR1CRFP. Transfected were assessed by flow cytometry, gated on MR1C expression using RFP (left), and then assessed for GFP expression, which was based on a dox-off control (second). Overlay histograms of MR1AGFP expression following MR1C (third) transfection as described in Fig. 6B with gMFI of MR1AGFP-negative condition reported. Doxycycline off transfection conditions are included as a control. Data are representative of at least 3 independent experiments with similar results. (F) ELISpot of cells transfected with pCI_MR1CRFP according to (B) and used as antigen presenting cells following infection with M.smegmatis at the indicated MOI (x-axis). Cells were incubated with a MAIT clone and IFN-γ production was measured. Data are pooled from 3 independent experiments performed in duplicate and error bars represent mean and standard deviation from the mean (n = 6).